424B3: Prospectus filed pursuant to Rule 424(b)(3)
Published on November 29, 2016
Filed Pursuant to Rule 424(b)(3)
Registration No. 333-204905
Prospectus Supplement No. 23
(To Prospectus dated October 14, 2015)

4,000,000 shares of common stock issuable upon the
exercise of the 4,000,000 outstanding Class A warrants
and
2,000,000 shares of common stock issuable upon the
exercise of the 4,000,000 outstanding Class B warrants
This prospectus supplement No. 23 supplements the prospectus dated October 14, 2015 filed pursuant to Rule 424(b)(4) by Cerecor Inc. (the “Company” or “we”), as supplemented by the prospectus supplement No. 1 dated October 20, 2015, the prospectus supplement No. 2 dated November 13, 2015, the prospectus supplement No. 3 dated November 23, 2015, the prospectus supplement No. 4 dated December 17, 2015, the prospectus supplement No. 5 dated December 21, 2015, the prospectus supplement No. 6 dated December 29, 2015, the prospectus supplement No. 7 dated January 5, 2016, the prospectus supplement No. 8 dated January 12, 2016, the prospectus supplement No. 9 dated January 19, 2016, the prospectus supplement No. 10 dated February 2, 2016, the prospectus supplement No. 11 dated April 11, 2016, the prospectus supplement No. 12 dated May 25, 2016, the prospectus supplement No. 13 dated May 26, 2016, the prospectus supplement No. 14 dated May 26, 2016, the prospectus supplement No. 15 dated July 20, 2016, the prospectus supplement No. 16 dated August 15, 2016, the prospectus supplement No. 17 dated August 29, 2016, the prospectus supplement No. 18 dated September 6, 2016, the prospectus supplement No. 19 dated September 12, 2016, the prospectus supplement No. 20 dated September 21, 2016, the prospectus supplement No. 21 dated September 26, 2016 and the prospectus supplement No. 22 dated November 8, 2016, each filed pursuant to Rule 424(b)(3) by the Company (collectively, the “Prospectus”). Pursuant to the Prospectus, this prospectus supplement relates to the continuous offering of 4,000,000 shares of common stock underlying our Class A warrants and 2,000,000 shares of our common stock underlying Class B warrants. Each warrant was a component of a unit that we issued in our initial public offering, which closed on October 20, 2015. The components of the units began to trade separately on November 13, 2015. Each Class A warrant became exercisable on the date when the units detached and the components began to trade separately and will expire on October 20, 2018, or earlier upon redemption. Each Class B warrant became exercisable on the date the units detached and the components began to trade separately and will expire on April 20, 2017.
This prospectus supplement incorporates into our Prospectus the information contained in our attached Current Report on Form 8-K, which was filed with the Securities and Exchange Commission on November 29, 2016.
You should read this prospectus supplement in conjunction with the Prospectus, including any supplements and amendments thereto. This prospectus supplement is qualified by reference to the Prospectus except to the extent that the information in this prospectus supplement supersedes the information contained in the Prospectus.
This prospectus supplement is not complete without, and may not be delivered or utilized except in connection with, the Prospectus, including any supplements and amendments thereto.
Our common stock, the Class A warrants and the Class B warrants are traded on The NASDAQ Capital Market under the symbols “CERC,” “CERCW,” and “CERCZ,” respectively.
AN INVESTMENT IN OUR SECURITIES INVOLVES A HIGH DEGREE OF RISK. SEE THE
SECTION ENTITLED “RISK FACTORS” BEGINNING ON PAGE 16 OF THE PROSPECTUS
FOR A DISCUSSION OF INFORMATION THAT SHOULD BE CAREFULLY CONSIDERED IN CONNECTION WITH AN INVESTMENT IN OUR SECURITIES
Neither the Securities and Exchange Commission nor any state securities commission has
approved or disapproved of these securities or determined if this Prospectus is truthful
or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus supplement is November 29, 2016
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 29, 2016
Cerecor Inc.
(Exact name of Registrant as Specified in Its Charter)
|
Delaware |
|
001-37590 |
|
45-0705648 |
|
(State or Other Jurisdiction |
|
(Commission |
|
(IRS Employer Identification No.) |
|
400 E. Pratt Street Suite 606 Baltimore, Maryland |
|
21202 |
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (410) 522-8707
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 8.01.Other Events.
On November 29, 2016, Cerecor Inc. (the “Company”) issued a press release in connection with the reporting of the results from its Phase 2 clinical trial with CERC-301 as an oral, adjunctive treatment of major depressive disorder. A copy of the press release is attached hereto as Exhibit 99.1 and is incorporated herein by reference.
The press release also announced that the Company will hold a conference call and webcast on November 29, 2016 at 5:00 p.m. Eastern time to discuss the CERC-301 clinical trial. A copy of the slides that will be presented on the webcast is attached hereto as Exhibit 99.2 and incorporated herein by reference.
Item 9.01.Financial Statements and Exhibits.
|
Exhibit No. |
|
Description |
|
|
|
|
|
99.1 |
|
Press Release, dated November 29, 2016, entitled “Cerecor Reports Top-Line Data from CERC-301 Phase 2 Study for Major Depressive Disorder.” |
|
|
|
|
|
99.2 |
|
Slides to be presented on webcast on November 29, 2016. |
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
Cerecor Inc. |
|
|
|
|
|
|
|
|
|
By: |
/s/ Uli Hacksell |
|
|
|
|
Uli Hacksell |
|
|
|
|
President and Chief Executive Officer |
Date: November 29, 2016
3
EXHIBIT INDEX
|
Exhibit No. |
|
Description |
|
|
|
|
|
99.1 |
Press Release, dated November 29, 2016, entitled “Cerecor Reports Top-Line Data from CERC-301 Phase 2 Study for Major Depressive Disorder.” |
|
|
|
|
|
|
99.2 |
|
Slides to be presented on webcast on November 29, 2016. |
4
Exhibit 99.1

Cerecor Reports Top-Line Data from CERC-301 Phase 2 Study for Major Depressive Disorder
CERC-301 misses primary endpoint but the 20 mg dose shows clinically meaningful efficacy signals at day 2
Management to Hold Conference Call and Webcast Today at 5:00 pm ET
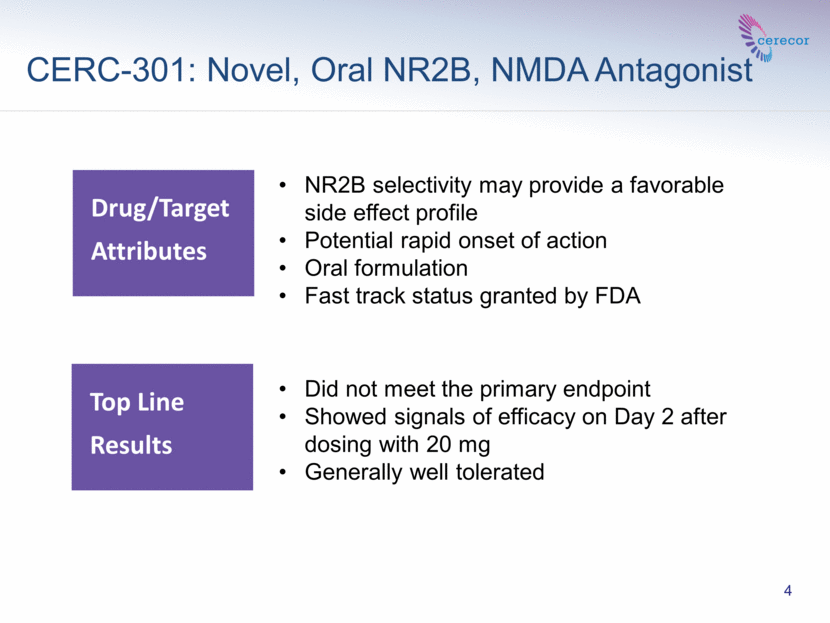
BALTIMORE--(Marketwired)--November 29, 2016--Cerecor Inc. (NASDAQ: CERC), a clinical-stage biopharmaceutical company developing treatments to make a difference in the lives of patients with neurological and psychiatric disorders, today announced top-line clinical results from its major depressive disorder (MDD) Phase 2 clinical trial (Clin301-203) of adjunctive treatment of CERC-301, an oral, NR2B specific, NMDA receptor antagonist. Overall, the trial failed to demonstrate efficacy on the primary endpoint for mean improvement in Bech-6, a subset of the Hamilton Depression Scale (HDRS-17), averaged over days 2 and 4 post dose. However, the study showed signals for the CERC-301 20 mg dose group at Day 2, at pre-specified secondary endpoints, indicating a potentially clinically-meaningful effect, though not statistically significant, on the Bech-6 and HDRS-17.
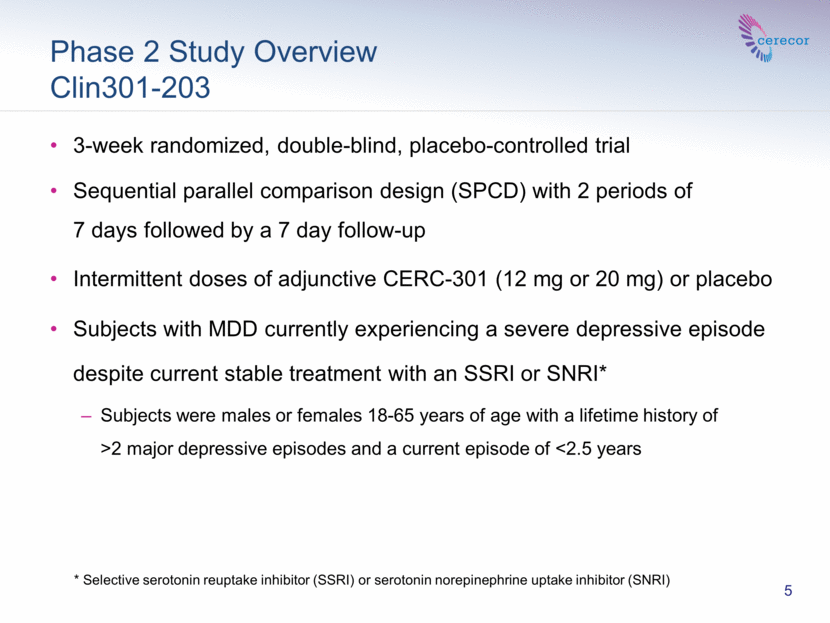
The trial was a 3-week randomized, double-blind, placebo-controlled, sequential parallel comparison design (SPCD) study of intermittent doses of adjunctive CERC-301 (12 mg or 20 mg) or placebo in the treatment of subjects with MDD who have not adequately responded to antidepressant therapy, either a selective serotonin reuptake inhibitor (SSRI) or a serotonin norepinephrine uptake inhibitor (SNRI).
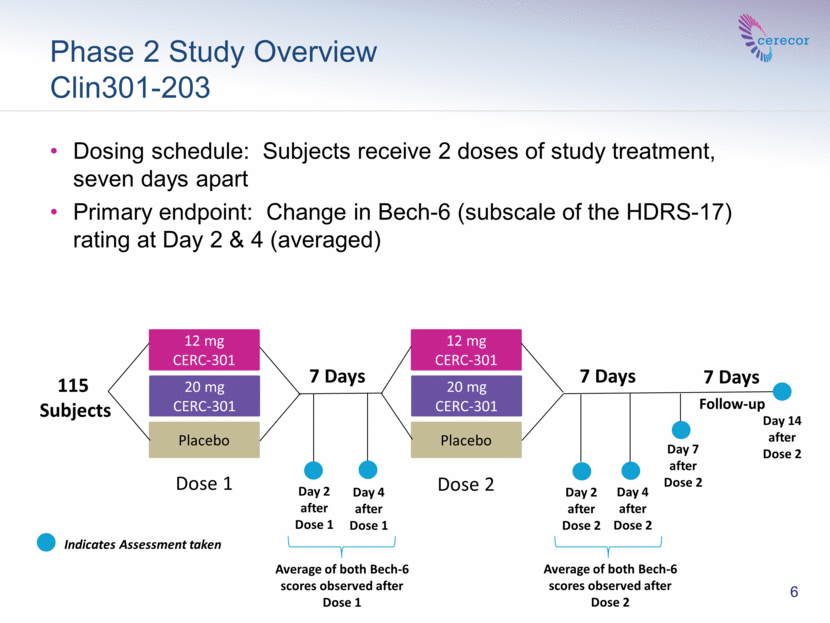
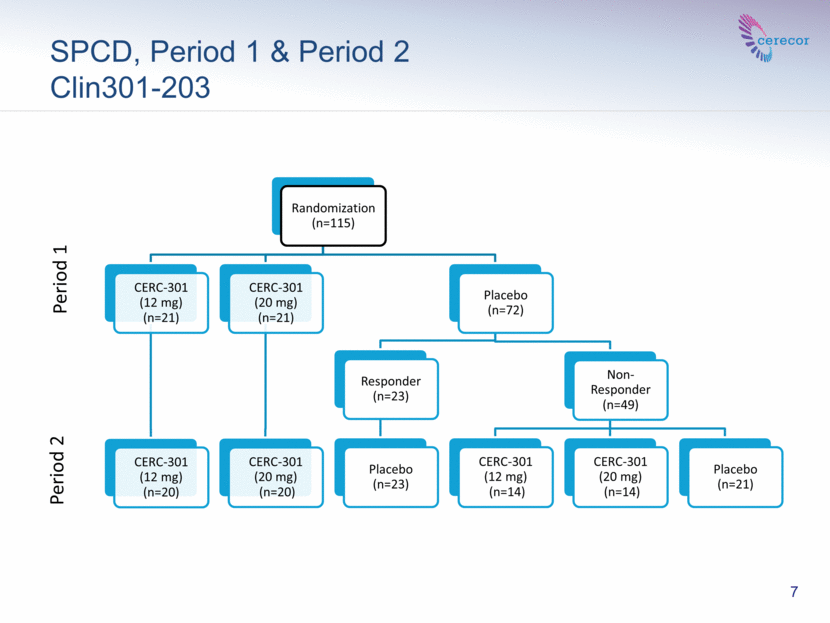
The study randomized 115 subjects. The study was conducted in two sequential one week periods. Placebo non-responders in Period 1 were re-randomized to either study drug or placebo in Period 2. Subjects received treatment at the beginning of each period. The results of Periods 1 and 2 were averaged. The study included a one-week follow-up.
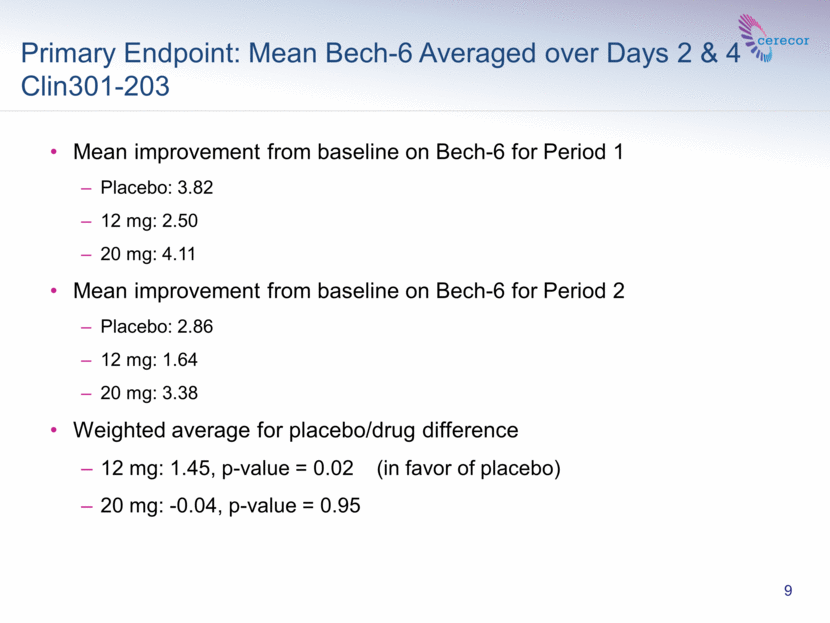
In this SPCD designed study, the mean improvement from baseline on the Bech-6 scale averaged over Days 2 and 4 post treatment for Period 1 was 3.82 for placebo, 2.50 for the 12 mg dose and 4.11 for the 20 mg dose, and for Period 2 was 2.86 for placebo, 1.64 for the 12mg dose and 3.38 for the 20 mg dose. The weighted average for the difference in placebo and drug improvement (placebo minus drug) was +1.45 and -0.04 for 12 mg and 20 mg CERC-301, respectively.
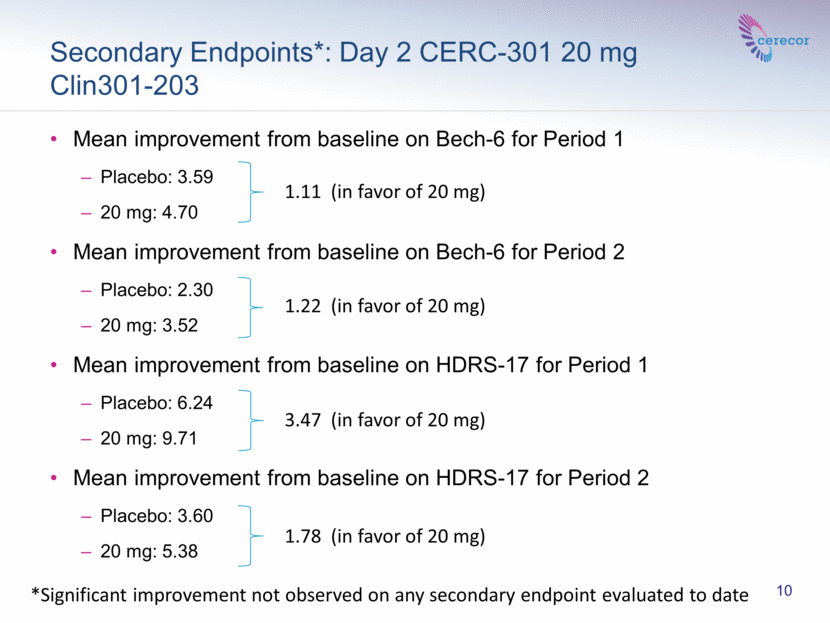
In a pre-specified analysis of the mean improvement from baseline on the Bech-6 at Day 2 for Period 1 was 3.59 for placebo, 4.71 for 20 mg; and for Period 2, 2.30 for placebo and 3.52 for 20 mg. In another pre-specified analysis of the mean improvement from baseline on the HDRS-17 at Day 2 for Period 1 was 6.24 for placebo and 9.71 for 20 mg; and for Period 2, 3.60 for placebo and 5.38 for 20 mg.
Significant improvement was not observed on the other secondary endpoints evaluated to date.
Consistent with previous trials, CERC-301 was generally well-tolerated with no SAEs reported and no discontinuations due to AEs. The most commonly reported adverse events in the study were increased blood pressure, dizziness, somnolence and paresthesia.
"While the trial failed to achieve the primary efficacy endpoint, we note that these results suggest a potentially clinically meaningful treatment effect in the 20 mg dose at Day 2,” added Ronald Marcus, M.D., Chief Medical Officer and Head of Regulatory Affairs at Cerecor.
“Based on this well conducted and controlled clinical trial, we continue to believe that adjunctive CERC-301 may have the potential to reduce depressive symptoms very rapidly with the added patient convenience of oral dosing," said Dr. Uli Hacksell, President and Chief Executive Officer of Cerecor. “We intend to more fully assess the results from this trial as we continue to receive the remaining data sets over the coming weeks and will announce planned next steps for CERC-301 at a later time."
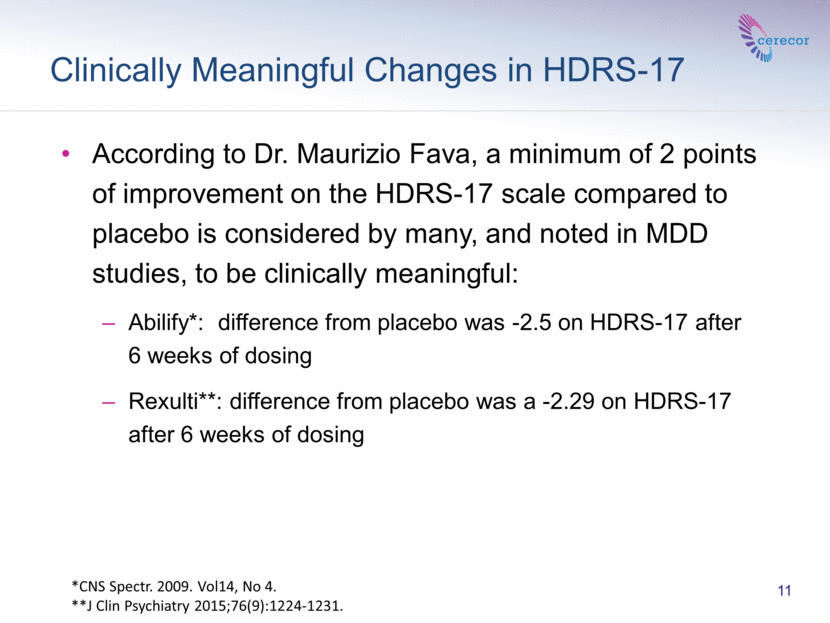
“From a clinician’s perspective, these data warrant additional clinical testing to fully explore the potential for this compound to treat MDD,” said Dr. Maurizio Fava, Director, Division of Clinical Research of the Massachusetts General Hospital Research Institute & Executive Vice Chair, Department of Psychiatry. “I would note that a minimum of 2 points of improvement on the HDRS-17 scale compared to placebo is considered by many, and noted in MDD studies, to be clinically meaningful.”
Cerecor intends to present additional data from this trial at scientific meetings in 2017.
Conference Call
Cerecor’s management team will host a conference call and webcast today, November 29, 2016 at 5:00 p.m. ET to discuss these top-line results. Presentation slides will be available via the webcast link. A question and answer session will follow Management's remarks. To participate on the live call, please dial 877-407-2985 (domestic) or +1-201-378-4915 (international), approximately 5 to 10 minutes ahead of the start of the call.
A live audio webcast of the call will be available via the "Investor Relations" page of the Cerecor website, www.cerecor.com. Please log on through Cerecor's website approximately 10 minutes prior to the scheduled start time. A replay of the webcast will be archived on Cerecor's website for 90 days following the call.
About CERC-301
CERC-301 is an oral, NR2B selective, NMDA receptor antagonist being developed as an adjunctive treatment of MDD. CERC‑301 may have the potential to be a first-in-class medication that may significantly reduce depressive symptoms in a matter of days. Based on the signal of potential clinical meaningfulness shown in Phase 2 testing, we are currently assessing future development.
About Cerecor
Cerecor is a clinical-stage biopharmaceutical company developing innovative drug candidates to make a difference in the lives of patients with neurological and psychiatric disorders. In addition to CERC-301, Cerecor is currently pursuing the development of CERC-501, which is also a clinical Phase 2-stage product candidate, as well as two earlier stage programs.
CERC-501 is a potent and selective kappa opioid receptor antagonist that is currently in a Phase 2 clinical trial for smoking cessation that is expected to provide top-line data in December 2016. In addition to Cerecor’s Phase 2 trial, three externally-funded clinical trials are being conducted to evaluate the use of CERC-501 in treating depressive symptoms, stress related smoking relapse and cocaine addiction. One study is being conducted under the auspices of the National Institute of Mental Health, the second is a collaboration between Cerecor and Yale University with funding from the National Institutes of Health and the third is being conducted at Rockefeller University Hospital with funding from a private foundation. Cerecor intends to begin Phase 2 testing of CERC-501 for the treatment of adjunctive MDD.
CERC-611 is a potent and selective Transmembrane AMPA Receptor Regulatory Proteins (“TARP”)-γ8-dependent α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (“AMPA”) receptor antagonist, which we plan to develop as an adjunctive therapy for the treatment of partial-onset seizures with or without secondarily generalized seizures in patients with epilepsy. We expect to file an investigational new drug application with the FDA and thereafter commence Phase 1 development in 2017.
Cerecor’s brain penetrant catechol‑O‑methyltransferase inhibitors, including CERC-406, are in preclinical development and may have potential procognitive activity.
For Investors:
Mariam E. Morris, Chief Financial Officer
(443) 304-8002.
For Media:
David Schull or Maggie Beller
Russo Partners LLC
646-942-5631
Maggie.beller@russopartnersllc.com
Forward-Looking Statements
This press release may include forward-looking statements made pursuant to the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that are not historical facts. Such forward-looking statements are subject to significant risks and uncertainties that are subject to change based on various factors (many of which are beyond Cerecor's control), which could cause actual results to differ from the forward-looking statements. Such statements may include, without limitation, statements with respect to Cerecor's plans, objectives, projections, expectations and intentions and other statements identified by words such as "projects," "may," "will," "could," "would," "should," "continue," "seeks," "aims," "predicts," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions (including their use in the negative), or by discussions of future matters such as the development of product candidates or products, potential benefits of product candidates, the expected timing of the commencement of clinical trials, the expected timing of data from clinical trials, technology enhancements and other statements that are not historical. These statements are based upon the current beliefs and expectations of Cerecor's management but are subject to significant risks and uncertainties, including those detailed in Cerecor's filings with the Securities and Exchange Commission. Actual results may differ from those set forth in the forward-looking statements. Except as required by applicable law, Cerecor expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Cerecor's expectations with respect thereto or any change in events, conditions or circumstances on which any statement is based.
Exhibit 99.2
|
|
Clin301-203 Phase 2 Topline MDD Data Conference Call November 29, 2016 NASDAQ:CERC www.cerecor.com |
|
|
Welcome Mariam E. Morris, CPA CFO Introduction Uli Hacksell, Ph.D. CEO, President and Chairman CERC-301 Phase 2 Trial Design and Results Ronald Marcus, M.D. CMO and Head, Regulatory Affairs Next Steps/Close Uli Hacksell, Ph.D. CEO, President and Chairman Q&A Cerecor Management Agenda 2 |
|
|
Forward-looking Statements This presentation contains forward-looking statements, including statements about our plans to develop and potentially commercialize our product candidates, our ongoing and planned clinical trials and preclinical studies for our product candidates, the timing of the availability of data from our clinical trials, the timing of and our ability to obtain and maintain regulatory approvals for our product candidates, the potential clinical utility and benefits of our product candidates, our intellectual property position and potential acquisitions, in-licenses or collaborations. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. These and other risks concerning our business are described in additional detail in our Quarterly Report on Form 10-Q filed with the SEC on November 8, 2016 and our other Periodic and Current Reports filed with the SEC. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Further, the information contained in this presentation speaks only as the date hereof. While we may elect to update the information in this presentation in the future, we disclaim any obligation to do so except to the extent required by applicable law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. 3 |
|
|
CERC-301: Novel, Oral NR2B, NMDA Antagonist Drug/Target Attributes NR2B selectivity may provide a favorable side effect profile Potential rapid onset of action Oral formulation Fast track status granted by FDA 4 Did not meet the primary endpoint Showed signals of efficacy on Day 2 after dosing with 20 mg Generally well tolerated Top Line Results |
|
|
Phase 2 Study Overview Clin301-203 3-week randomized, double-blind, placebo-controlled trial Sequential parallel comparison design (SPCD) with 2 periods of 7 days followed by a 7 day follow-up Intermittent doses of adjunctive CERC-301 (12 mg or 20 mg) or placebo Subjects with MDD currently experiencing a severe depressive episode despite current stable treatment with an SSRI or SNRI* Subjects were males or females 18-65 years of age with a lifetime history of >2 major depressive episodes and a current episode of <2.5 years 5 * Selective serotonin reuptake inhibitor (SSRI) or serotonin norepinephrine uptake inhibitor (SNRI) |
|
|
Phase 2 Study Overview Clin301-203 Dosing schedule: Subjects receive 2 doses of study treatment, seven days apart Primary endpoint: Change in Bech-6 (subscale of the HDRS-17) rating at Day 2 & 4 (averaged) 7 Days 7 Days Day 2 after Dose 1 Day 4 after Dose 1 Follow-up 12 mg CERC-301 20 mg CERC-301 Placebo 115 Subjects 12 mg CERC-301 20 mg CERC-301 Placebo Day 2 after Dose 2 Day 4 after Dose 2 Day 7 after Dose 2 7 Days Dose 1 Dose 2 6 Indicates Assessment taken Average of both Bech-6 scores observed after Dose 1 Average of both Bech-6 scores observed after Dose 2 Day 14 after Dose 2 |
|
|
SPCD, Period 1 & Period 2 Clin301-203 7 Period 1 Period 2 Randomization (n=115) CERC-301 (12 mg) (n=21) CERC-301 (12 mg) (n=20) CERC-301 (20 mg) (n=21) CERC-301 (20 mg) (n=20) Placebo (n=72) Responder (n=23) Placebo (n=23) Non-Responder (n=49) CERC-301 (12 mg) (n=14) CERC-301 (20 mg) (n=14) Placebo (n=21) |
|
|
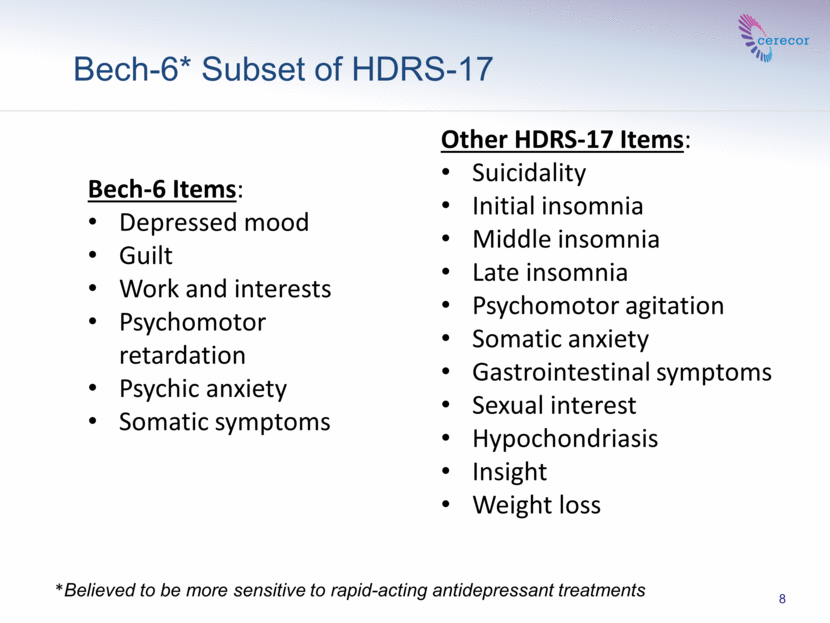
8 *Believed to be more sensitive to rapid-acting antidepressant treatments Bech-6* Subset of HDRS-17 Bech-6 Items: Depressed mood Guilt Work and interests Psychomotor retardation Psychic anxiety Somatic symptoms Other HDRS-17 Items: Suicidality Initial insomnia Middle insomnia Late insomnia Psychomotor agitation Somatic anxiety Gastrointestinal symptoms Sexual interest Hypochondriasis Insight Weight loss |
|
|
Primary Endpoint: Mean Bech-6 Averaged over Days 2 & 4 Clin301-203 9 Mean improvement from baseline on Bech-6 for Period 1 Placebo: 3.82 12 mg: 2.50 20 mg: 4.11 Mean improvement from baseline on Bech-6 for Period 2 Placebo: 2.86 12 mg: 1.64 20 mg: 3.38 Weighted average for placebo/drug difference 12 mg: 1.45, p-value = 0.02 (in favor of placebo) 20 mg: -0.04, p-value = 0.95 |
|
|
Secondary Endpoints*: Day 2 CERC-301 20 mg Clin301-203 Mean improvement from baseline on Bech-6 for Period 1 Placebo: 3.59 20 mg: 4.70 Mean improvement from baseline on Bech-6 for Period 2 Placebo: 2.30 20 mg: 3.52 Mean improvement from baseline on HDRS-17 for Period 1 Placebo: 6.24 20 mg: 9.71 Mean improvement from baseline on HDRS-17 for Period 2 Placebo: 3.60 20 mg: 5.38 3.47 (in favor of 20 mg) 1.78 (in favor of 20 mg) 1.11 (in favor of 20 mg) 1.22 (in favor of 20 mg) 10 *Significant improvement not observed on any secondary endpoint evaluated to date |
|
|
Clinically Meaningful Changes in HDRS-17 11 According to Dr. Maurizio Fava, a minimum of 2 points of improvement on the HDRS-17 scale compared to placebo is considered by many, and noted in MDD studies, to be clinically meaningful: Abilify*: difference from placebo was -2.5 on HDRS-17 after 6 weeks of dosing Rexulti**: difference from placebo was a -2.29 on HDRS-17 after 6 weeks of dosing *CNS Spectr. 2009. Vol14, No 4. **J Clin Psychiatry 2015;76(9):1224-1231. |
|
|
Safety and Tolerability Clin301-203 CLIN 301-203 Phase 2 12 Generally well-tolerated No SAEs reported No discontinuations due to AEs Most commonly reported: Increased blood pressure Dizziness Somnolence Paresthesia |
|
|
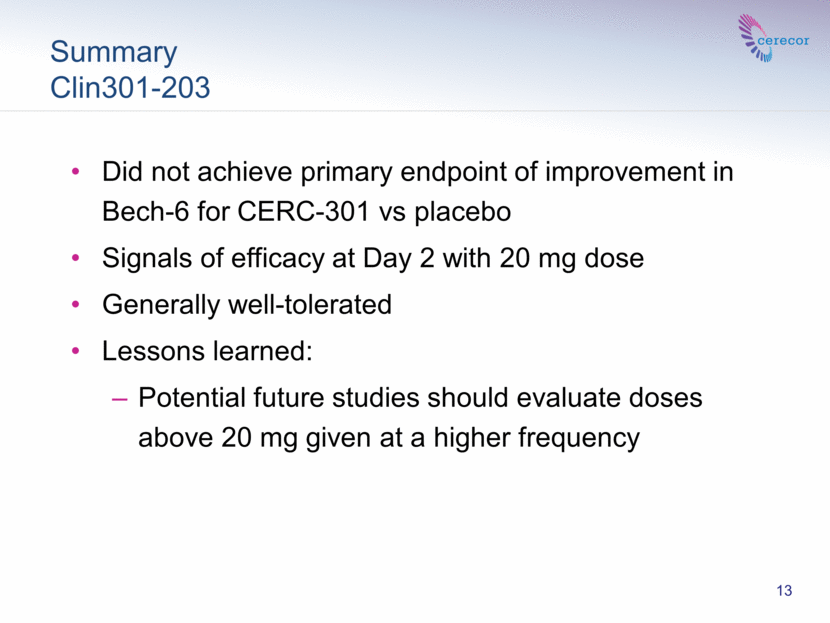
Summary Clin301-203 CLIN 301-203 Phase 2 13 Did not achieve primary endpoint of improvement in Bech-6 for CERC-301 vs placebo Signals of efficacy at Day 2 with 20 mg dose Generally well-tolerated Lessons learned: Potential future studies should evaluate doses above 20 mg given at a higher frequency |
|
|
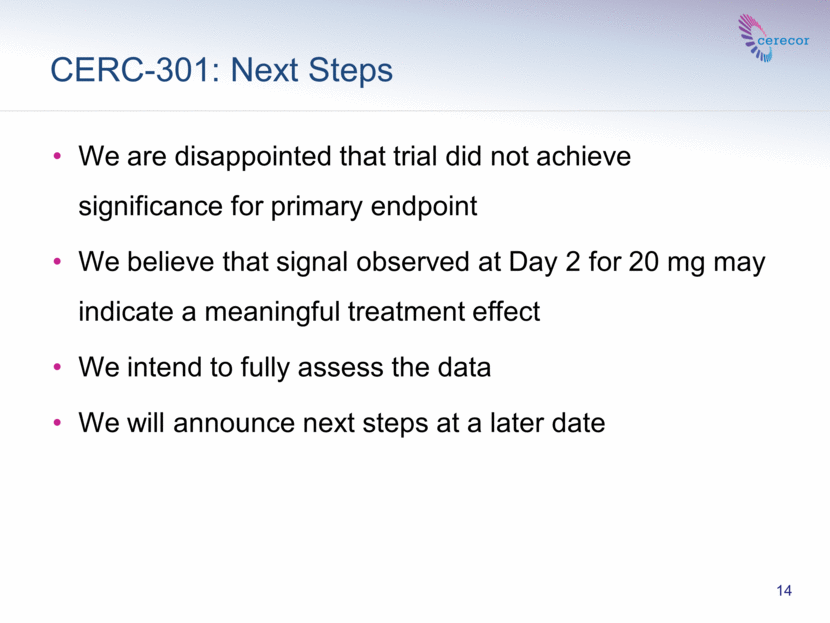
CERC-301: Next Steps 14 We are disappointed that trial did not achieve significance for primary endpoint We believe that signal observed at Day 2 for 20 mg may indicate a meaningful treatment effect We intend to fully assess the data We will announce next steps at a later date |
|
|
Q&A |