000153412012/312025Q1FALSENoneNoneNoneNonehttp://fasb.org/us-gaap/2024#DerivativeLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2024#DerivativeLiabilitiesNoncurrenthttp://www.avalotherapeutics.com/20250331#PropertyPlantAndEquipmentAndOperatingLeaseRightOfUseAssetAfterAccumulatedDepreciationAndAmortizationhttp://www.avalotherapeutics.com/20250331#PropertyPlantAndEquipmentAndOperatingLeaseRightOfUseAssetAfterAccumulatedDepreciationAndAmortizationhttp://fasb.org/us-gaap/2024#AccruedLiabilitiesAndOtherLiabilitieshttp://fasb.org/us-gaap/2024#AccruedLiabilitiesAndOtherLiabilitieshttp://fasb.org/us-gaap/2024#OtherLiabilitiesNoncurrenthttp://fasb.org/us-gaap/2024#OtherLiabilitiesNoncurrentP1YP6Mxbrli:sharesiso4217:USDiso4217:USDxbrli:sharesxbrli:pureavtx:propertyavtx:class_of_stockavtx:unitavtx:segment00015341202025-01-012025-03-3100015341202025-05-0700015341202025-03-3100015341202024-12-310001534120us-gaap:SeriesDPreferredStockMember2025-03-310001534120us-gaap:SeriesDPreferredStockMember2024-12-310001534120us-gaap:SeriesEPreferredStockMember2024-12-310001534120us-gaap:SeriesEPreferredStockMember2025-03-310001534120us-gaap:SeriesCPreferredStockMember2025-03-310001534120us-gaap:SeriesCPreferredStockMember2024-12-3100015341202024-01-012024-03-310001534120us-gaap:CommonStockMember2024-12-310001534120us-gaap:PreferredStockMemberus-gaap:SeriesCPreferredStockMember2024-12-310001534120us-gaap:AdditionalPaidInCapitalMember2024-12-310001534120us-gaap:RetainedEarningsMember2024-12-310001534120us-gaap:CommonStockMember2025-01-012025-03-310001534120us-gaap:PreferredStockMemberus-gaap:SeriesCPreferredStockMember2025-01-012025-03-310001534120us-gaap:AdditionalPaidInCapitalMember2025-01-012025-03-310001534120us-gaap:RetainedEarningsMember2025-01-012025-03-310001534120us-gaap:CommonStockMember2025-03-310001534120us-gaap:PreferredStockMemberus-gaap:SeriesCPreferredStockMember2025-03-310001534120us-gaap:AdditionalPaidInCapitalMember2025-03-310001534120us-gaap:RetainedEarningsMember2025-03-3100015341202023-12-310001534120us-gaap:CommonStockMember2023-12-310001534120us-gaap:PreferredStockMemberus-gaap:SeriesCPreferredStockMember2023-12-310001534120us-gaap:AdditionalPaidInCapitalMember2023-12-310001534120us-gaap:RetainedEarningsMember2023-12-310001534120us-gaap:CommonStockMember2024-01-012024-03-310001534120us-gaap:CommonStockMemberavtx:AlmataBioTransactionMember2024-01-012024-03-310001534120us-gaap:AdditionalPaidInCapitalMemberavtx:AlmataBioTransactionMember2024-01-012024-03-310001534120avtx:AlmataBioTransactionMember2024-01-012024-03-310001534120us-gaap:SeriesCPreferredStockMemberavtx:AlmataBioTransactionMember2024-01-012024-03-310001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PrivatePlacementMember2024-01-012024-03-310001534120us-gaap:SeriesDPreferredStockMemberus-gaap:PrivatePlacementMember2024-01-012024-03-310001534120us-gaap:SeriesEPreferredStockMemberus-gaap:PrivatePlacementMember2024-01-012024-03-310001534120us-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-310001534120us-gaap:RetainedEarningsMember2024-01-012024-03-3100015341202024-03-310001534120us-gaap:CommonStockMember2024-03-310001534120us-gaap:PreferredStockMemberus-gaap:SeriesCPreferredStockMember2024-03-310001534120us-gaap:AdditionalPaidInCapitalMember2024-03-310001534120us-gaap:RetainedEarningsMember2024-03-310001534120srt:RevisionOfPriorPeriodReclassificationAdjustmentMember2024-12-310001534120srt:RevisionOfPriorPeriodReclassificationAdjustmentMember2024-03-310001534120avtx:AlmataBioTransactionMember2024-03-272024-03-270001534120us-gaap:SeriesCPreferredStockMemberavtx:AlmataTransactionAndMarch2024FinancingMember2024-03-272024-03-270001534120us-gaap:CommonStockMemberavtx:AlmataBioTransactionMember2024-03-272024-03-270001534120avtx:AlmataBioTransactionMember2024-03-282024-03-280001534120avtx:AlmataBioTransactionMember2024-04-012024-04-300001534120avtx:MilestoneOneMemberavtx:AlmataBioTransactionMember2024-03-280001534120avtx:MilestoneTwoMemberavtx:AlmataBioTransactionMember2024-03-280001534120avtx:MilestoneOneMemberavtx:AlmataBioTransactionMember2024-10-012024-10-310001534120avtx:AlmataBioTransactionMember2024-03-310001534120avtx:AlmataBioTransactionMember2024-03-270001534120avtx:MillipredMember2021-07-012021-07-010001534120us-gaap:LicenseMember2025-01-012025-03-310001534120us-gaap:LicenseMember2024-01-012024-03-310001534120avtx:EmployeeConsultantsAndDirectorsStockOptionsMember2025-01-012025-03-310001534120avtx:EmployeeConsultantsAndDirectorsStockOptionsMember2024-01-012024-03-310001534120avtx:WarrantCommonStockMember2025-01-012025-03-310001534120avtx:WarrantCommonStockMember2024-01-012024-03-310001534120us-gaap:SeriesCPreferredStockMember2025-01-012025-03-310001534120us-gaap:SeriesCPreferredStockMember2024-01-012024-03-310001534120us-gaap:RestrictedStockUnitsRSUMember2025-01-012025-03-310001534120us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-03-310001534120us-gaap:SeriesCPreferredStockMember2025-03-310001534120us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2025-03-310001534120us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2025-03-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2025-03-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2025-03-310001534120us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001534120us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-12-310001534120us-gaap:FairValueInputsLevel3Member2024-12-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2025-01-012025-03-310001534120us-gaap:FairValueInputsLevel3Member2025-01-012025-03-310001534120us-gaap:FairValueInputsLevel3Member2025-03-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:WarrantMember2023-12-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2023-12-310001534120us-gaap:FairValueInputsLevel3Member2023-12-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:WarrantMember2024-01-012024-03-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-01-012024-03-310001534120us-gaap:FairValueInputsLevel3Member2024-01-012024-03-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:WarrantMember2024-03-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2024-03-310001534120us-gaap:FairValueInputsLevel3Member2024-03-310001534120avtx:ESTherapeuticsMemberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2022-10-012022-12-310001534120avtx:AVTX501Member2022-12-310001534120avtx:MilestoneOneMemberavtx:AVTX007Member2022-12-310001534120avtx:MilestoneTwoMemberavtx:AVTX007Member2022-12-310001534120avtx:AVTX611Member2022-12-310001534120avtx:AVTX501AndAVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2022-10-012022-12-310001534120avtx:AVTX501Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2022-10-012022-12-310001534120avtx:AVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2022-10-012022-12-310001534120avtx:AVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2025-03-310001534120avtx:AVTX007Member2025-01-012025-03-310001534120avtx:AVTX501AndAVTX007Memberus-gaap:DerivativeFinancialInstrumentsLiabilitiesMember2025-01-012025-03-310001534120avtx:AVTX501Member2025-03-310001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberavtx:MeasurementInputProbabilityOfSuccessMemberavtx:AVTX501Member2022-12-310001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberavtx:MeasurementInputSalesForecastPeakMemberavtx:AVTX007Member2022-10-012022-12-310001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberavtx:MeasurementInputProbabilityOfSuccessMemberavtx:AVTX007Member2022-12-310001534120us-gaap:DerivativeFinancialInstrumentsLiabilitiesMemberus-gaap:MeasurementInputExpectedTermMemberavtx:AVTX007Member2022-10-012022-12-310001534120us-gaap:WarrantMemberus-gaap:PrivatePlacementMember2025-03-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:WarrantMember2025-01-012025-03-310001534120us-gaap:PreferredStockMemberus-gaap:PrivatePlacementMember2025-01-012025-03-310001534120stpr:PAus-gaap:BuildingMember2025-03-310001534120stpr:PAus-gaap:BuildingMember2025-01-012025-03-310001534120stpr:MDus-gaap:BuildingMember2025-03-310001534120stpr:MDus-gaap:BuildingMember2025-01-012025-03-310001534120stpr:MDus-gaap:BuildingMember2024-10-012024-12-310001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PrivatePlacementMember2024-03-282024-03-280001534120us-gaap:WarrantMemberus-gaap:PrivatePlacementMember2024-03-282024-03-280001534120us-gaap:PreferredStockMemberus-gaap:PrivatePlacementMember2024-03-282024-03-280001534120us-gaap:PrivatePlacementMember2024-03-282024-03-280001534120us-gaap:PrivatePlacementMember2024-10-012024-12-310001534120us-gaap:SeriesCPreferredStockMemberus-gaap:PrivatePlacementMember2024-08-132024-08-130001534120us-gaap:CommonStockMember2024-08-132024-08-130001534120us-gaap:CommonStockMember2024-10-012024-12-310001534120us-gaap:SeriesCPreferredStockMember2024-10-012024-12-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:WarrantMember2025-03-310001534120us-gaap:FairValueInputsLevel3Memberus-gaap:WarrantMember2024-10-012024-12-310001534120us-gaap:AdditionalPaidInCapitalMemberus-gaap:PrivatePlacementMember2024-10-012024-12-310001534120avtx:AlmataTransactionAndMarch2024FinancingMember2025-03-310001534120us-gaap:SeriesCPreferredStockMemberavtx:AlmataTransactionAndMarch2024FinancingMember2025-03-310001534120us-gaap:SeriesCPreferredStockMemberavtx:AlmataBioTransactionAndMarch2024FinancingMember2025-03-310001534120avtx:AlmataTransactionAndMarch2024FinancingMember2024-10-012024-12-310001534120us-gaap:SeriesCPreferredStockMemberavtx:AlmataTransactionAndMarch2024FinancingMember2024-12-310001534120us-gaap:AdditionalPaidInCapitalMemberus-gaap:SeriesCPreferredStockMemberavtx:AlmataBioTransactionAndMarch2024FinancingMember2024-12-310001534120us-gaap:SeriesCPreferredStockMember2025-01-012025-03-310001534120avtx:ATMAgreementMember2023-05-042023-05-040001534120avtx:ATMAgreementMember2023-08-012023-08-310001534120avtx:ATMAgreementMember2025-01-012025-03-310001534120avtx:CommonStockWarrantsExpirationJune2031Memberus-gaap:CommonClassAMember2025-03-310001534120avtx:A2016ThirdAmendedPlanMember2025-01-012025-03-310001534120avtx:A2016FourthAmendedPlanMember2025-01-012025-01-010001534120avtx:A2016ThirdAmendedPlanMember2025-03-310001534120us-gaap:StockOptionMemberavtx:A2016ThirdAmendedPlanMember2025-01-012025-03-310001534120us-gaap:ShareBasedPaymentArrangementEmployeeMemberavtx:A2016ThirdAmendedPlanMemberus-gaap:StockOptionMembersrt:MaximumMember2025-01-012025-03-310001534120avtx:A2016ThirdAmendedPlanMemberus-gaap:StockOptionMembersrt:DirectorMembersrt:MinimumMember2025-01-012025-03-310001534120avtx:A2016ThirdAmendedPlanMemberus-gaap:StockOptionMembersrt:DirectorMembersrt:MaximumMember2025-01-012025-03-310001534120us-gaap:ResearchAndDevelopmentExpenseMember2025-01-012025-03-310001534120us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-03-310001534120us-gaap:GeneralAndAdministrativeExpenseMember2025-01-012025-03-310001534120us-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-03-310001534120avtx:ServiceBasedOptionsMember2024-12-310001534120avtx:ServiceBasedOptionsMember2024-01-012024-12-310001534120avtx:ServiceBasedOptionsMember2025-01-012025-03-310001534120avtx:ServiceBasedOptionsMember2025-03-310001534120avtx:ServiceBasedOptionsMemberavtx:ChiefStrategyOfficerMember2025-01-012025-01-010001534120avtx:ServiceBasedOptionsMemberavtx:ChiefStrategyOfficerMember2025-01-282025-01-280001534120avtx:ServiceBasedOptionsMember2024-01-012024-03-310001534120avtx:ServiceBasedOptionsMembersrt:MinimumMember2025-03-310001534120avtx:ServiceBasedOptionsMembersrt:MaximumMember2025-03-310001534120avtx:ServiceBasedOptionsMembersrt:MinimumMember2025-01-012025-03-310001534120avtx:ServiceBasedOptionsMembersrt:MaximumMember2025-01-012025-03-310001534120us-gaap:RestrictedStockUnitsRSUMember2024-12-310001534120us-gaap:RestrictedStockUnitsRSUMember2025-01-012025-03-310001534120us-gaap:RestrictedStockUnitsRSUMember2025-03-310001534120us-gaap:RestrictedStockUnitsRSUMember2024-08-132024-08-130001534120us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-03-310001534120us-gaap:EmployeeStockMember2016-04-052016-04-050001534120us-gaap:EmployeeStockMember2016-04-050001534120us-gaap:EmployeeStockMember2025-01-012025-01-010001534120us-gaap:EmployeeStockMember2025-01-012025-03-310001534120avtx:LillyLicenseAgreementMemberavtx:AVTX009LillyLicenseAgreementMember2024-03-270001534120avtx:LillyLicenseAgreementMembersrt:MinimumMemberavtx:AVTX009LillyLicenseAgreementMember2024-03-270001534120avtx:LillyLicenseAgreementMembersrt:MaximumMemberavtx:AVTX009LillyLicenseAgreementMember2024-03-270001534120avtx:LillyLicenseAgreementMemberavtx:AVTX009LillyLicenseAgreementMember2024-03-272024-03-270001534120avtx:LillyLicenseAgreementMemberavtx:AVTX009LillyLicenseAgreementMember2025-01-012025-03-310001534120avtx:LillyLicenseAgreementMemberavtx:AVTX009LillyLicenseAgreementMember2025-03-310001534120avtx:KyowaKirinCoLtdKKCMemberavtx:AVTX002KKCLicenseAgreementMember2021-03-250001534120avtx:MilestoneOneMemberavtx:KyowaKirinCoLtdKKCMemberavtx:AVTX002KKCLicenseAgreementMember2021-03-250001534120avtx:MilestoneTwoMemberavtx:KyowaKirinCoLtdKKCMemberavtx:AVTX002KKCLicenseAgreementMember2021-03-250001534120us-gaap:ContractTerminationMemberavtx:KyowaKirinCoLtdKKCMembersrt:MinimumMember2021-03-252021-03-250001534120us-gaap:ContractTerminationMemberavtx:KyowaKirinCoLtdKKCMembersrt:MaximumMember2021-03-252021-03-250001534120avtx:KyowaKirinCoLtdKKCMemberavtx:AVTX002KKCLicenseAgreementMember2025-01-012025-03-310001534120avtx:KyowaKirinCoLtdKKCMemberavtx:AVTX002KKCLicenseAgreementMember2025-03-310001534120avtx:CHOPLicenseAgreementMemberavtx:AVTX002KKCLicenseAgreementMember2020-02-030001534120avtx:CHOPLicenseAgreementMemberavtx:AVTX002KKCLicenseAgreementMember2020-01-012020-12-310001534120avtx:CHOPLicenseAgreementMemberavtx:AVTX002KKCLicenseAgreementMember2025-03-310001534120avtx:CHOPLicenseAgreementMember2020-02-032020-02-030001534120us-gaap:ContractTerminationMemberavtx:CHOPLicenseAgreementMember2020-02-032020-02-030001534120avtx:SanfordBurnhamPrebysMedicalDiscoveryInstituteMemberavtx:AVTX008SanfordBurnhamPrebysLicenseAgreementMember2021-06-210001534120avtx:SanfordBurnhamPrebysMedicalDiscoveryInstituteMemberavtx:AVTX008SanfordBurnhamPrebysLicenseAgreementMember2021-06-300001534120avtx:MilestoneOneMemberavtx:SanfordBurnhamPrebysMedicalDiscoveryInstituteMemberavtx:AVTX008SanfordBurnhamPrebysLicenseAgreementMember2021-06-210001534120avtx:MilestoneTwoMemberavtx:SanfordBurnhamPrebysMedicalDiscoveryInstituteMemberavtx:AVTX008SanfordBurnhamPrebysLicenseAgreementMember2021-06-210001534120avtx:SanfordBurnhamPrebysMedicalDiscoveryInstituteMembersrt:MinimumMemberavtx:AVTX008SanfordBurnhamPrebysLicenseAgreementMember2021-06-210001534120avtx:SanfordBurnhamPrebysMedicalDiscoveryInstituteMembersrt:MaximumMemberavtx:AVTX008SanfordBurnhamPrebysLicenseAgreementMember2021-06-210001534120us-gaap:ContractTerminationMemberavtx:SanfordBurnhamPrebysMedicalDiscoveryInstituteMember2021-06-212021-06-210001534120avtx:SanfordBurnhamPrebysMedicalDiscoveryInstituteMemberavtx:AVTX008SanfordBurnhamPrebysLicenseAgreementMember2025-01-012025-03-310001534120avtx:SanfordBurnhamPrebysMedicalDiscoveryInstituteMemberavtx:AVTX008SanfordBurnhamPrebysLicenseAgreementMember2025-03-310001534120avtx:AstellasPharmaIncAstellasMemberavtx:AVTX006AstellasLicenseAgreementMember2019-07-150001534120avtx:AstellasPharmaIncAstellasMemberavtx:AVTX006Member2019-07-152019-07-150001534120avtx:AstellasPharmaIncAstellasMember2019-07-152019-07-150001534120avtx:AstellasPharmaIncAstellasMemberavtx:AVTX006AstellasLicenseAgreementMember2025-01-012025-03-310001534120avtx:AstellasPharmaIncAstellasMemberavtx:AVTX006AstellasLicenseAgreementMember2025-03-310001534120avtx:AltoMemberavtx:AVTX301OutLicenseMember2021-05-280001534120avtx:AVTX301OutLicenseMember2021-05-282021-05-280001534120avtx:AltoMemberavtx:AVTX301OutLicenseMember2025-03-310001534120avtx:MilestoneOneMemberavtx:ESMemberavtx:AVTX406LicenseAssignmentMember2021-06-090001534120avtx:MilestoneTwoMemberavtx:ESMemberavtx:AVTX406LicenseAssignmentMember2021-06-090001534120avtx:ESMemberavtx:AVTX406LicenseAssignmentMember2025-03-310001534120avtx:AUGTherapeuticsLLCMemberavtx:AVTX800SeriesAssetSaleMemberavtx:PurchaseAgreementMember2023-10-272023-10-270001534120avtx:AUGTherapeuticsLLCMemberavtx:AVTX800SeriesAssetSaleMemberavtx:PurchaseAgreementMember2023-10-270001534120avtx:MilestoneTwoMemberavtx:AVTX009Member2024-04-300001534120avtx:MilestoneThreeMemberavtx:AVTX009Member2024-10-012024-10-3100015341202019-07-012019-07-310001534120avtx:TRISPharmaMemberavtx:KarbinalAgreementMember2018-01-012018-12-310001534120avtx:ReportableSegmentMember2025-01-012025-03-310001534120avtx:ReportableSegmentMember2024-01-012024-03-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-Q
| | | | | |
☑
| QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
for the quarterly period ended March 31, 2025 |
| OR |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
COMMISSION FILE NUMBER: 001-37590
AVALO THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| | | | | |
Delaware (State of incorporation) | 45-0705648 (I.R.S. Employer Identification No.) |
1500 Liberty Ridge Drive, Suite 321 Wayne, Pennsylvania 19087 (Address of principal executive offices) | (410) 522-8707 (Registrant’s telephone number, including area code) |
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol | Name of each exchange on which registered |
| Common Stock, $0.001 par value | AVTX | Nasdaq Capital Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b‑2 of the Exchange Act.
| | | | | | | | |
Large accelerated filer ☐ | | Accelerated filer ☐ |
Non-accelerated filer ☑ | | Smaller reporting company ☑ |
Emerging growth company ☐ | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b‑2 of the Exchange Act). Yes ☐ No ☑
As of May 7, 2025, the registrant had 10,827,620 shares of common stock outstanding.
AVALO THERAPEUTICS, INC.
FORM 10-Q
For the Quarter Ended March 31, 2025
TABLE OF CONTENTS
PART I - FINANCIAL INFORMATION
Item 1. Financial Statements.
AVALO THERAPEUTICS, INC. and SUBSIDIARIES
Condensed Consolidated Balance Sheets
(In thousands, except share and per share data)
| | | | | | | | | | | | | | |
| | March 31, 2025 | | December 31, 2024 |
| | (unaudited) | | |
| Assets | | | | |
| Current assets: | | | | |
| Cash and cash equivalents | | $ | 125,046 | | | $ | 134,546 | |
| | | | |
| | | | |
| | | | |
| Prepaid expenses and other current assets | | 1,833 | | | 4,325 | |
| Restricted cash, current portion | | 62 | | | 19 | |
| Total current assets | | 126,941 | | | 138,890 | |
| Property and equipment, net | | 949 | | | 1,209 | |
| Goodwill | | 10,502 | | | 10,502 | |
| Restricted cash, net of current portion | | 131 | | | 131 | |
| Total assets | | $ | 138,523 | | | $ | 150,732 | |
| Liabilities, mezzanine equity and stockholders’ equity | | | | |
| Current liabilities: | | | | |
| Accounts payable | | $ | 681 | | | $ | 283 | |
| Accrued expenses and other current liabilities | | 4,574 | | | 6,317 | |
Derivative liability, current | | 360 | | | 360 | |
| | | | |
| | | | |
| Total current liabilities | | 5,615 | | | 6,960 | |
| Royalty obligation | | 2,000 | | | 2,000 | |
| Deferred tax liability, net | | 278 | | | 270 | |
Derivative liability, non-current | | 7,740 | | | 8,120 | |
| Other long-term liabilities | | 275 | | | 350 | |
| Total liabilities | | 15,908 | | | 17,700 | |
| Mezzanine equity: | | | | |
Series D Preferred Stock—$0.001 par value; 1 share of Series D Preferred Stock authorized at March 31, 2025 and December 31, 2024; 1 share of Series D Preferred Stock issued and outstanding at March 31, 2025 and December 31, 2024 | | — | | | — | |
Series E Preferred Stock—$0.001 par value; 1 share of Series E Preferred Stock authorized at March 31, 2025 and December 31, 2024; 1 share of Series E Preferred Stock issued and outstanding at March 31, 2025 and December 31, 2024 | | — | | | — | |
| Stockholders’ equity: | | | | |
Common stock—$0.001 par value; 200,000,000 shares authorized at March 31, 2025 and December 31, 2024; 10,827,620 and 10,471,934 shares issued and outstanding at March 31, 2025 and December 31, 2024, respectively | | 11 | | | 10 | |
Series C Preferred Stock—$0.001 par value; 34,326 shares of Series C Preferred Stock authorized at March 31, 2025 and December 31, 2024; 24,696 and 24,896 shares of Series C Preferred Stock issued and outstanding at March 31, 2025 and December 31, 2024, respectively | | — | | | — | |
| Additional paid-in capital | | 506,016 | | | 503,285 | |
| Accumulated deficit | | (383,412) | | | (370,263) | |
| Total stockholders’ equity | | 122,615 | | | 133,032 | |
| Total liabilities, mezzanine equity and stockholders’ equity | | $ | 138,523 | | | $ | 150,732 | |
See accompanying notes to the unaudited condensed consolidated financial statements.
AVALO THERAPEUTICS, INC. and SUBSIDIARIES
Condensed Consolidated Statements of Operations and Comprehensive Loss (Unaudited)
(In thousands, except per share data)
| | | | | | | | | | | | | | | | | | |
| | Three Months Ended | | |
| | | March 31, | | |
| | | 2025 | | 2024 | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Operating expenses: | | | | | | | | |
| Cost of product sales | | — | | | (80) | | | | | |
| Research and development | | 9,123 | | | 2,116 | | | | | |
| General and administrative | | 5,546 | | | 3,193 | | | | | |
| Acquired in-process research and development | | — | | | 27,538 | | | | | |
| | | | | | | | |
| Total operating expenses | | 14,669 | | | 32,767 | | | | | |
| Loss from operations | | (14,669) | | | (32,767) | | | | | |
| Other income (expense): | | | | | | | | |
| Change in fair value of derivative liability | | 380 | | | (120) | | | | | |
| Interest income, net | | 1,148 | | | 100 | | | | | |
| Excess of initial warrant fair value over private placement proceeds | | — | | | (79,276) | | | | | |
| | | | | | | | |
| Private placement transaction costs | | — | | | (9,220) | | | | | |
| | | | | | | | |
| Total other income (expense), net | | 1,528 | | | (88,516) | | | | | |
| Loss before taxes | | (13,141) | | | (121,283) | | | | | |
| Income tax expense | | 8 | | | 7 | | | | | |
| Net loss and comprehensive loss | | $ | (13,149) | | | $ | (121,290) | | | | | |
| | | | | | | | |
| | | | | | | | |
Weighted average common shares outstanding | | 10,514,901 | | | 859,381 | | | | | |
| Net loss per share of common stock, basic and diluted | | $ | (1.25) | | | $ | (141.14) | | | | | |
| | | | | | | | |
See accompanying notes to the unaudited condensed consolidated financial statements.
AVALO THERAPEUTICS, INC. and SUBSIDIARIES
Condensed Consolidated Statements of Mezzanine and Stockholders’ Equity (Unaudited)
(In thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Mezzanine Preferred Stock | | | Common stock | | Series C Preferred Stock | | | | Additional paid-in | | Accumulated | Total stockholders’ |
| | Shares | Amount | | | Shares | | Amount | | Shares | | Amount | | | | | | capital | | deficit | | equity |
Three Months Ended March 31, 2025 | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2024 | 2 | | $ | — | | | | 10,471,934 | | | $ | 10 | | | 24,896 | | | $ | — | | | | | | | $ | 503,285 | | | $ | (370,263) | | | $ | 133,032 | |
| Issuance of common stock in exchange for retirement of Series C Preferred Stock | — | | — | | | | 200,000 | | | 1 | | | (200) | | | — | | | | | | | — | | | — | | | 1 | |
Vesting of Restricted Stock Units net of shares withheld for taxes | — | | — | | | | 155,686 | | | — | | | — | | | — | | | | | | | (510) | | | | | (510) | |
| Stock-based compensation | — | | — | | | | — | | | — | | | — | | | — | | | | | | | 3,241 | | | — | | | 3,241 | |
Net loss | — | | — | | | | — | | | — | | | — | | | — | | | | | | | — | | | (13,149) | | | (13,149) | |
| Balance, March 31, 2025 | 2 | | $ | — | | | | 10,827,620 | | | $ | 11 | | | 24,696 | | | $ | — | | | | | | | $ | 506,016 | | | $ | (383,412) | | | $ | 122,615 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Mezzanine Preferred Stock | | | Common stock | | | | Series C Preferred Stock | | Additional paid-in | | Accumulated | Total stockholders’ | | |
| | Shares | Amount | | | Shares | | Amount | | | | | | Shares | | Amount | | capital | | deficit | | equity | | |
| Three Months Ended March 31, 2024 | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2023 | — | | $ | — | | | | 801,746 | | | $ | 1 | | | | | | | — | | | $ | — | | | $ | 342,437 | | | $ | (335,134) | | | $ | 7,304 | | | |
| Impact of reverse split fractional share round-up | — | | — | | | | 60,779 | | | — | | | | | | | — | | | — | | | — | | | — | | | — | | | |
Issuance of common stock pursuant to AlmataBio Transaction | — | | — | | | | 171,605 | | | — | | | | | | | — | | | — | | | 815 | | | — | | | 815 | | | |
Issuance of Series C Preferred Stock pursuant to AlmataBio Transaction | 2,412 | | 11,457 | | | | — | | | — | | | | | | | — | | | — | | | — | | | — | | | — | | | |
| Issuance of Series C Preferred Stock in private placement | 19,946 | | — | | | | — | | | — | | | | | | | — | | | — | | | — | | | — | | | — | | | |
| Issuance of Series D Preferred Stock in private placement | 1 | | — | | | | — | | | — | | | | | | | — | | | — | | | — | | | — | | | — | | | |
| Issuance of Series E Preferred Stock in private placement | 1 | | — | | | | — | | | — | | | | | | | — | | | — | | | — | | | — | | | — | | | |
| Stock-based compensation | — | | — | | | | — | | | — | | | | | | | — | | | — | | | 629 | | | — | | | 629 | | | |
| Net loss | — | | — | | | | — | | | — | | | | | | | — | | | — | | | — | | | (121,290) | | | (121,290) | | | |
| Balance, March 31, 2024 | 22,360 | | $ | 11,457 | | | | 1,034,130 | | | $ | 1 | | | | | | | — | | | $ | — | | | $ | 343,881 | | | $ | (456,424) | | | $ | (112,542) | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
AVALO THERAPEUTICS, INC. and SUBSIDIARIES
Condensed Consolidated Statements of Cash Flows (Unaudited)
(Amounts in thousands) | | | | | | | | | | | | | | |
| | | Three Months Ended March 31, |
| | | 2025 | | 2024 |
| Operating activities | | | | |
Net loss | | $ | (13,149) | | | $ | (121,290) | |
| Adjustments to reconcile net loss used in operating activities: | | | | |
| Depreciation and amortization | | 135 | | | 34 | |
| Stock-based compensation | | 3,241 | | | 629 | |
| Acquired in-process research and development | | — | | | 27,538 | |
| Excess of initial warrant fair value over private placement proceeds | | — | | | 79,276 | |
| | | | |
| Transaction costs paid pursuant to private placement | | — | | | 7,013 | |
| | | | |
| Transaction costs payable upon exercise of warrants issued in private placement | | — | | | 1,734 | |
| Cash payable to tax authorities related to withholding shares to satisfy RSU withholding obligations | | (509) | | | |
| Change in fair value of derivative liability | | (380) | | | 120 | |
| | | | |
| Deferred taxes | | 8 | | | 7 | |
| Changes in assets and liabilities: | | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| Prepaid expenses and other assets | | 2,492 | | | (53) | |
| | | | |
| Lease incentive | | — | | | 158 | |
| Accounts payable | | 398 | | | 470 | |
| | | | |
| | | | |
| | | | |
| Accrued expenses and other liabilities | | (1,700) | | | (1,652) | |
| Lease liability, net | | 7 | | | (186) | |
| Net cash used in operating activities | | (9,457) | | | (6,202) | |
| Investing activities | | | | |
Cash assumed from AlmataBio Transaction | | — | | | 356 | |
| | | | |
| | | | |
| | | | |
Net cash provided by investing activities | | — | | | 356 | |
| Financing activities | | | | |
| Proceeds from Notes and warrants, net of debt issuance costs paid | | — | | | — | |
| | | | |
| | | | |
| Proceeds from private placement investment, gross | | — | | | 115,625 | |
| Transaction costs paid pursuant to private placement | | — | | | (7,013) | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| Net cash provided by financing activities | | — | | | 108,612 | |
(Decrease) increase in cash, cash equivalents and restricted cash | | (9,457) | | | 102,766 | |
| Cash, cash equivalents, and restricted cash at beginning of period | | 134,696 | | | 7,546 | |
| Cash, cash equivalents, and restricted cash at end of period | | $ | 125,239 | | | $ | 110,312 | |
| | | | |
| | | | |
| | | | |
| Supplemental disclosures of non-cash activities | | | | |
| Cash payable to tax authorities related to withholding shares to satisfy RSU withholding obligations | | $ | (509) | | | $ | — | |
Issuance of common stock and Series C Preferred Stock pursuant to AlmataBio Transaction | | $ | — | | | $ | 12,272 | |
| | | | |
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported within the condensed consolidated balance sheets that sum to the total of the same such amounts shown in the condensed consolidated statements of cash flows (in thousands):
| | | | | | | | | | | | | | |
| | March 31, |
| | 2025 | | 2024 |
| Cash and cash equivalents | | $ | 125,046 | | | $ | 110,177 | |
| Restricted cash, current | | 62 | | | 4 | |
| Restricted cash, non-current | | 131 | | | 131 | |
| Total cash, cash equivalents and restricted cash | | $ | 125,239 | | | 110,312 | |
See accompanying notes to the unaudited condensed consolidated financial statements.
AVALO THERAPEUTICS, INC. and SUBSIDIARIES
Notes to Unaudited Condensed Consolidated Financial Statements
1. Business
Avalo Therapeutics, Inc. (the “Company,” “Avalo” or “we”) is a clinical stage biotechnology company focused on the treatment of immune dysregulation. Avalo’s lead asset is AVTX-009, an anti-IL-1β monoclonal antibody (“mAb”), targeting inflammatory diseases.
Avalo was incorporated in Delaware and commenced operation in 2011, and completed its initial public offering in October 2015.
Liquidity
Since inception, we have incurred significant operating and cash losses from operations. We have primarily funded our operations to date through sales of equity securities, out-licensing transactions and sales of assets.
For the three months ended March 31, 2025, Avalo generated net loss of $13.1 million and negative cash flows from operations of $9.5 million. As of March 31, 2025, Avalo had $125.0 million in cash and cash equivalents.
In accordance with Accounting Standards Codification Topic 205-40, Presentation of Financial Statements - Going Concern, the Company evaluated its ability to continue as a going concern within one year after the date that the accompanying condensed consolidated financial statements are issued. Based on our current operating plans, we expect that our existing cash and cash equivalents are sufficient to fund operations for at least twelve months from the filing date of this Quarterly Report on Form 10-Q. The Company closely monitors its cash and cash equivalents and seeks to balance the level of cash and cash equivalents with our projected needs to allow us to withstand periods of uncertainty relative to the availability of funding on favorable terms. We may satisfy any future cash needs through sales of equity securities under the Company’s at-the-market program or other equity financings, out-licensing transactions, strategic alliances/collaborations, sale of programs, and/or mergers and acquisitions. There can be no assurance that any financing or business development initiatives can be realized by the Company, or if realized, what the terms may be. To the extent that we raise capital through the sale of equity, the ownership interest of our existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our stockholders. Further, if the Company raises additional funds through collaborations, strategic alliances or licensing arrangements with third parties, the Company might have to relinquish valuable rights to its technologies, future revenue streams, research programs or product candidates.
2. Basis of Presentation and Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (the “FASB”). The unaudited condensed consolidated financial statements have been prepared on the basis of continuity of operations, realization of assets, and the satisfaction of liabilities in the ordinary course of business.
In the opinion of management, the accompanying unaudited condensed consolidated financial statements include all adjustments, consisting of normal recurring adjustments, which are necessary to present fairly the Company’s financial position, results of operations, and cash flows. The condensed consolidated balance sheet at December 31, 2024 has been derived from audited financial statements at that date. The interim results of operations are not necessarily indicative of the results that may occur for the full fiscal year. Certain information and footnote disclosure normally included in the financial statements prepared in accordance with GAAP have been condensed or omitted pursuant to instructions, rules, and regulations prescribed by the United States Securities and Exchange Commission (“SEC”).
The Company believes that the disclosures provided herein are adequate to make the information presented not misleading when these unaudited condensed consolidated financial statements are read in conjunction with the December 31, 2024 audited consolidated financial statements.
In the first quarter of 2025, the Company concluded that it would include other receivables with the prepaid and other current assets line in the Company’s unaudited condensed consolidated balance sheets and statement of cash flows. The Company reclassified $0.6 million and $0.1 million from other receivables to prepaid and other current receivables as of December 31, 2024 and for the three months ended March 31, 2024, respectively, to conform with the current period presentation.
Unless otherwise indicated, all amounts in the following tables are in thousands except share and per share amounts.
Significant Accounting Policies
During the three months ended March 31, 2025, there were no significant changes to the Company’s summary of significant accounting policies contained in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, as filed with the SEC on March 20, 2025.
3. Asset Acquisition
AlmataBio Transaction
On March 27, 2024, the Company acquired AVTX-009, an anti-IL-1β mAb, through a merger of AlmataBio, Inc. (“AlmataBio”) with and into its wholly owned subsidiary (the “AlmataBio Transaction”). The Company’s acquisition of AlmataBio was structured as a stock-for-stock transaction whereby all outstanding equity interests in AlmataBio were exchanged in a merger for a combination of the Company’s common stock and shares of the Company’s non-voting convertible preferred stock (the “Series C Preferred Stock”), resulting in the issuance of 171,605 shares of Company common stock and 2,412 shares of Series C Preferred Stock. Upon Company stockholder approval on August 13, 2024 and subject to beneficial ownership limitations, 2,063 shares of Series C Preferred Stock issued to former AlmataBio stockholders automatically converted into 2,062,930 shares of common stock.
In addition to the shares issued, a cash payment of $7.5 million was due to the former AlmataBio stockholders upon the closing of a private placement. The private placement closed on March 28, 2024 and the Company paid the $7.5 million in April 2024. The Company is also required to pay potential development milestone payments to the former AlmataBio stockholders, including $5.0 million due upon the first patient dosed in a Phase 2 trial in patients with hidradenitis suppurativa (“HS”) for AVTX-009, and $15.0 million due upon the first patient dosed in a Phase 3 trial for AVTX-009, both of which are payable in cash or Avalo stock at the election of the former AlmataBio stockholders, subject to the terms and conditions of the definitive merger agreement. In October 2024, the first development milestone was met and the Company paid the $5.0 million cash payment.
The Company is the acquiring company for accounting purposes. In connection with the AlmataBio Transaction, substantially all of the consideration paid is allocable to the fair value of acquired in-process research and development (“IPR&D”), specifically AVTX-009, and as such the acquisition is treated as an asset acquisition. The Company initially recognized AlmataBio’s assets and liabilities by allocating the accumulated cost of the acquisition based on their relative fair values, as estimated by management. The net assets acquired as of the transaction date have been combined with the assets, liabilities, and results of operations of the Company on consummation of the AlmataBio Transaction. In accordance with ASC 730, Research and Development, the portion of the consideration allocated to the acquired IPR&D, specifically AVTX-009, based on its relative fair value, is included as an operating expense as there is no alternative future use.
Below is a summary of the total consideration, assets acquired and the liabilities assumed in connection with the AlmataBio Transaction (in thousands):
| | | | | | | | | | |
| | Three Months Ended March 31, 2024 |
Stock consideration1 | | $ | 12,272 | | | |
Milestone payment due upon close of private placement investment2 | | 7,500 | | | |
Milestone payment due upon first patient dosed in a Phase 2 trial2 | | 5,000 | | | |
| Transaction costs | | 2,402 | | | |
| Total GAAP Purchase Price at Close | | $ | 27,174 | | | |
| | | | |
| Acquired IPR&D | | $ | 27,538 | | | |
| Cash | | 356 | | | |
| Accrued expenses and other current liabilities | | (720) | | | |
| Total net assets acquired and liabilities assumed | | $ | 27,174 | | | |
1 Equal to the aggregate common stock issued of 171,605 and the aggregate shares of Series C Preferred Stock issued of 2,412 (as-convertible to 2,412,000 shares of common stock), multiplied by the Company’s closing stock price of $4.75 on March 27, 2024. On August 13, 2024 upon Company stockholder approval and subject to beneficial ownership limitations, 2,063 of the 2,412 shares of Series C Preferred Stock were converted into 2,062,930 shares of common stock.
2 Avalo deemed these milestones probable and estimable as of the transaction close date and therefore included them as part of the GAAP purchase price at close. The milestone payment due upon the close of the private placement was paid in April 2024. The milestone payment due upon the first patient dosed in a Phase 2 trial was paid in October 2024.
4. Revenue
The Company’s license and supply agreement for Millipred®, an oral prednisolone indicated across a wide variety of inflammatory conditions, expired, as planned, on September 30, 2023. Avalo considered Millipred® a non-core asset. Historically, the Company sold Millipred® in the United States primarily through wholesale distributors, who accounted for substantially all of the Company’s net product revenues and trade receivables. The Company continues to monitor estimates for commercial liabilities for Millipred®, such as sales returns. As additional information becomes available, the Company could recognize expense (or a benefit) for differences between actuals or updated estimates to the reserves previously recognized.
Pursuant to the Millipred® license and supply agreement, Avalo was required to pay the supplier fifty percent of the net profit of the Millipred® product following each calendar quarter, with a $0.5 million quarterly minimum payment contingent on Avalo achieving certain net profit thresholds as stipulated in the agreement. The profit share commenced on July 1, 2021 and ended on September 30, 2023. Within twenty-five months of September 30, 2023, the net profit share is subject to a reconciliation process, where estimated deductions to arrive at net profit will be reconciled to actuals, which might result in Avalo owing additional amounts to the supplier or vice versa, which would be recognized in cost of product sales.
There was no gross revenue recognized from sales of prescription drugs for the three months ended March 31, 2025 and March 31, 2024.
5. Net Loss Per Share
The Company had two classes of stock outstanding during the three months ended March 31, 2025 and March 31, 2024, common stock and preferred stock. The Company computes net loss per share using the two-class method, as the Series C Preferred Stock participates in distributions with the Company’s common stock. The two-class method of computing net loss per share is an earnings allocation formula that determines net loss for common stock and any participating securities according to dividends declared and participation rights in undistributed earnings. As the Company is in a net loss position for the three months ended March 31, 2025 and 2024, the two-class method of calculating net loss per share results in no allocation of undistributed losses to participating securities.
Basic net loss per share for common stock is computed by dividing the sum of distributed and undistributed earnings by the weighted average number of shares outstanding for the period.
Diluted net loss per share includes the potential dilutive effect of common stock equivalents as if such securities were converted or exercised during the period, when the effect is dilutive. Common stock equivalents include: (i) outstanding stock options and restricted stock units, which are included under the “treasury stock method” when dilutive; (ii) common stock to be issued upon the exercise of outstanding warrants, which are included under the “treasury stock method” when dilutive, and (iii) preferred stock under the if-converted method. While the impact of these items are generally anti-dilutive during periods of net loss, the Company will determine whether the common stock equivalents should be included in diluted loss per share pursuant to sequencing rules.
The following tables set forth the computation of basic and diluted net loss per share of common stock for the three months ended March 31, 2025 and March 31, 2024 (in thousands, except share and per share amounts):
| | | | | | | | | | |
| | Three Months Ended March 31, 2025 | | |
| | | Common stock | | |
| | | | |
| | | | |
Net loss | | $ | (13,149) | | | |
| | | | |
| | | | |
| Weighted average shares | | 10,514,901 | | | |
Basic and diluted net loss per share | | $ | (1.25) | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | |
| | | | | | | | |
| | Three Months Ended March 31, 2024 |
| | | Common stock |
| | |
| Net loss | | $ | (121,290) | |
| Weighted average shares | | 859,381 | |
| Basic and diluted net loss per share | | $ | (141.14) | |
The following outstanding securities have been excluded from the computation of diluted weighted shares outstanding for the three months ended March 31, 2025 and 2024, as they could have been anti-dilutive:
| | | | | | | | | | | | | | | | | | |
| | | Three Months Ended | | |
| | March 31, | | |
| | | 20252 | | 2024 | | | | |
| Stock options | | 3,831,749 | | 7,543 | | | | |
Warrants on common stock | | 148 | | 11,969,063 | | | | |
Series C Preferred Stock (as-convertible to common stock)1 | | 24,695,920 | | 22,357,897 | | | | |
| Restricted Stock Units | | 421,397 | | — | | | | |
1 Each share of the Company’s Series C Preferred Stock is convertible to 1,000 shares of common stock, subject to certain beneficial ownership limitations.
2 Pursuant to the AlmataBio Transaction, the Company is required to pay potential development milestone payments to the former AlmataBio stockholders in cash or Avalo stock at the election of the former AlmataBio stockholders; refer to Notes 3 and 9 for more information. In the event of a settlement in shares, the number of Avalo shares delivered will vary based on the Company’s stock price. These additional shares are not included in the computation of basic and diluted net loss per share for the three months ended March 31, 2025 and 2024 pursuant to the guidance on contingently issuable shares.
6. Fair Value Measurements
ASC 820, Fair Value Measurements and Disclosures (“ASC 820”) defines fair value as the price that would be received to sell an asset, or paid to transfer a liability, in the principal or most advantageous market in an orderly transaction between market participants on the measurement date. The fair value standard also establishes a three‑level hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The valuation hierarchy is based upon the transparency of inputs to the valuation of an asset or liability on the measurement date. The three levels are defined as follows:
•Level 1—inputs to the valuation methodology are quoted prices (unadjusted) for an identical asset or liability in an active market.
•Level 2—inputs to the valuation methodology include quoted prices for a similar asset or liability in an active market or model‑derived valuations in which all significant inputs are observable for substantially the full term of the asset or liability.
•Level 3—inputs to the valuation methodology are unobservable and significant to the fair value measurement of the asset or liability.
The following table presents, for each of the fair value hierarchy levels required under ASC 820, the Company’s assets and liabilities that are measured at fair value on a recurring basis (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | March 31, 2025 |
| | | Fair Value Measurements Using |
| | | Quoted prices in active markets for identical assets | | Significant other observable inputs | | Significant unobservable inputs |
| | | (Level 1) | | (Level 2) | | (Level 3) |
| Assets | | | | | | |
| Investments in money market funds* | | $ | 74,097 | | | $ | — | | | $ | — | |
| Liabilities | | | | | | |
| | | | | | |
| Derivative liability | | $ | — | | | $ | — | | | $ | 8,100 | |
| | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2024 |
| | | Fair Value Measurements Using |
| | | Quoted prices in active markets for identical assets | | Significant other observable inputs | | Significant unobservable inputs |
| | | (Level 1) | | (Level 2) | | (Level 3) |
| Assets | | | | | | |
| Investments in money market funds* | | $ | 133,148 | | | $ | — | | | $ | — | |
| Liabilities | | | | | | |
| Derivative liability | | $ | — | | | $ | — | | | $ | 8,480 | |
*Investments in money market funds are reflected in cash and cash equivalents on the accompanying unaudited condensed consolidated balance sheets.
As of March 31, 2025 and December 31, 2024, the Company’s financial instruments included cash and cash equivalents, restricted cash, prepaid and other current assets, accounts payable, accrued expenses and other current liabilities, and derivative liability.
The carrying amounts reported in the accompanying unaudited condensed consolidated financial statements for cash and cash equivalents, restricted cash, accounts receivable, other receivables, prepaid and other current assets, accounts payable, and accrued expenses and other current liabilities approximate their respective fair values because of the short-term nature of these accounts.
Level 3 Valuation
The table presented below is a summary of changes in the fair value of the Company’s Level 3 valuations for the warrant liability and derivative liability for the three months ended March 31, 2025 and March 31, 2024:
| | | | | | | | | | | | | | | | |
| | | | | Derivative liability | | Total |
Balance at December 31, 2024 | | | | $ | 8,480 | | | $ | 8,480 | |
| | | | | | |
| Change in fair value | | | | (380) | | | (380) | |
Balance at March 31, 2025 | | | | $ | 8,100 | | | $ | 8,100 | |
| | | | | | | | | | | | | | | | | | | | |
| | | Warrant liability | | Derivative liability | | Total |
Balance at December 31, 2023 | | $ | — | | | $ | 5,550 | | | $ | 5,550 | |
| Initial valuation of warrant liability | | 194,901 | | | — | | | 194,901 | |
| Change in fair value | | — | | | 120 | | | 120 | |
Balance at March 31, 2024 | | $ | 194,901 | | | $ | 5,670 | | | $ | 200,571 | |
Derivative liability
In the fourth quarter of 2022, Avalo sold its economic rights to future milestone and royalty payments for previously out-licensed assets AVTX-501, AVTX-007, and AVTX-611 to ES Therapeutics, LLC (“ES”), an affiliate of Armistice Capital LLC (“Armistice”), in exchange for $5.0 million (the “ES Transaction”). At the time of the transaction, Armistice was a significant stockholder of the Company whose chief investment officer, Steven Boyd, and managing director, Keith Maher, served on Avalo’s Board until August 8, 2022. The ES Transaction was approved in accordance with Avalo’s related party transaction policy.
The economic rights sold include (a) rights to a milestone payment of $20.0 million upon the filing and acceptance of an NDA for AVTX-501 pursuant to an agreement with Janssen Pharmaceutics, Inc. (the “AVTX-501 Milestone”) and (b) rights to any future milestone payments and royalties relating to AVTX-007 under a license agreement with Apollo AP43 Limited (“Apollo”), including up to $6.25 million of development milestones, up to $67.5 million in sales-based milestones, and royalty payments over a ten year period of a low single digit percentage of annual net sales (which percentage increases to another low single digit percentage if annual net sales exceed a specified threshold) (the “AVTX-007 Milestones and Royalties”). In addition, Avalo waived all its rights to AVTX-611 sales-based payments of up to $20.0 million that were payable by ES.
The exchange of the economic rights of the AVTX-501 Milestone and AVTX-007 Milestones and Royalties for cash met the definition of a derivative instrument. The fair value of the derivative liability is determined using a combination of a scenario-based method and an option pricing method (implemented using a Monte Carlo simulation). The significant inputs including probabilities of success, expected timing, and forecasted sales as well as market-based inputs for volatility, risk-adjusted discount rates and allowance for counterparty credit risk are unobservable and based on the best information available to Avalo. Certain information used in the valuation is inherently limited in nature and could differ from Janssen and Apollo’s internal estimates.
The fair value of the derivative liability as of the transaction date was approximately $4.8 million, of which $3.5 million was attributable to the AVTX-501 Milestone and $1.3 million was attributable to the AVTX-007 Milestones and Royalties. Subsequent to the transaction date, at each reporting period, the derivative liability is remeasured at fair value. As of March 31, 2025, the fair value of the derivative liability was $8.1 million, all of which was attributable to the AVTX-007 Milestone and Royalties and $0.4 million of which was classified as a current liability with the remainder classified as a non-current liability. For the three months ended March 31, 2025, the $0.4 million change in fair value was recognized in other income (expense), net in the accompanying unaudited condensed consolidated statements of operations and comprehensive loss.
The fair value of the AVTX-501 Milestone was deemed to be $0.0 million, driven by less than 1% probability of success based on Avalo’s interpretation of a recent announcement from J&J noting the discontinuation of the aticaprant depression program (previously referred to as AVTX-501 by Avalo), which was the only indication that we are aware they were pursuing, paired with a lack of commitment to an alternative indication. The fair value of AVTX-007 Milestones and Royalties was primarily driven by sales forecasts with peak annual net sales reaching $1.8 billion in atopic dermatitis, which is a much larger market opportunity than adult-onset Still’s disease (the previous indication being pursued that was contemplated in valuations through the first quarter of 2024), an approximate 17% probability of success, and an estimated time to commercialization of approximately 5.8 years. We estimated these unobservable inputs based on limited publicly available information and therefore could differ from J&J’s and Apollo’s respective internal development plans, assessments of probability of success and other inputs of our fair value calculation. Any changes to these inputs may result in significant changes to the fair value measurement. Notably, the peak annual net sales forecast (for the AVTX-007 Milestones and Royalties) and the probability of success (for both the AVTX-501 Milestone and the AVTX-007 Milestone and Royalties) are the largest drivers of the fair value, so changes to either would likely result in significant changes to the fair value.
In the event that J&J and/or Apollo are required to make payment(s) to ES Therapeutics pursuant to the underlying agreements, Avalo will recognize revenue under its existing contracts with those customers for that amount when it is no longer probable there would be a significant revenue reversal with any differences between the fair value of the derivative liability related to that payment immediately prior to the revenue recognition and revenue recognized to be recorded as other expense. However, given Avalo is no longer entitled to collect these payments, the potential ultimate settlement of the payments in the future from J&J and/or Apollo to ES Therapeutics (and the future mark-to-market activity each reporting period) will not impact Avalo’s future cash flows.
Warrant liability
In March 2024, the Company closed a private placement investment with institutional investors in which the investors received shares of Series C Preferred Stock and warrants to purchase shares of Avalo’s common stock (or a number of shares of Series C Preferred Stock). Refer to Note 9 - Capital Structure and sub-header “March 2024 Financing” for more information.
The Company determined that the warrants did not satisfy the conditions to be accounted for as equity instruments. As the warrants did not meet the equity contract scope exception, the Company classified the warrants as a derivative liability upon issuance.
The Company’s warrant liability was measured at fair value on the issuance date and was measured at fair value each reporting period thereafter until the warrants were fully exercised in the fourth quarter of 2024. As of March 31, 2025, there were no warrants associated with the private placement outstanding and thus no corresponding warrant liability.
For the initial warrant valuation in the first quarter of 2024 and subsequent fair value measurement at each reporting period prior to exercises, the Company utilized the Black-Scholes option pricing model to measure fair value of the warrants, which required assumptions including the value of the stock on the measurement date, exercise price, expected term, expected volatility, and the risk-free interest rate. Certain assumptions, including the expected term and expected volatility, were subjective and required judgment. The warrant liability was classified as a Level 3 instrument as its value was based on unobservable market inputs.
The initial fair value measurement of the warrant liability was $194.9 million and exceeded the initial gross proceeds received from the private placement of $115.6 million, resulting in a $79.3 million loss at issuance of the excess of initial liability fair value. The warrants were fully exercised in the fourth quarter of 2024. Refer to Note 9 - Capital Structure for additional discussion regarding the issuance of the Series C Preferred Stock and common stock pursuant to the warrant exercises.
No changes in valuation techniques occurred during the three months ended March 31, 2025 and 2024. No transfers of assets between Level 1 and Level 2 of the fair value measurement hierarchy occurred during the three months ended March 31, 2025 and 2024.
7. Leases
Avalo currently occupies two leased properties, both of which serve as administrative office space. The Company determined that both of these leases are operating leases based on the lease classification test performed at lease commencement.
The initial annual base rent for the Company’s office located in Wayne (Chesterbrook), Pennsylvania is $0.2 million and the annual operating expenses are approximately $0.1 million. The annual base rent is subject to periodic increases of approximately 2.4% over the term of the lease. The lease has an initial term of 5.25 years from the lease commencement on December 1, 2021 and expires on February 28, 2027.
The annual base rent for the Company’s office located in Rockville, Maryland is $0.2 million, subject to annual 2.5% increases over the term of the lease. The lease provided for a rent abatement for a period of 12 months following the Company’s date of occupancy. The lease had an initial term of 10 years from the date the Company made its first annual fixed rent payment, which occurred in January 2020. In the fourth quarter of 2024, the Company paid the $0.3 million contractual early termination fee, electing to early-terminate the lease effective January 31, 2026, which represents the sixth anniversary of the first annual fixed rent payment. As a result of the early-termination, the lease liability and ROU asset were remeasured and reduced by $0.3 million.
The weighted average remaining term of the operating leases at March 31, 2025 was 1.7 years.
Supplemental balance sheet information related to the leased properties include (in thousands):
| | | | | | | | | | | | | | |
| | | As of |
| | | March 31, 2025 | | December 31, 2024 |
Right-of-use assets | | $ | 615 | | | $ | 741 | |
| | | | |
Lease liability, current | | $ | 526 | | | $ | 568 | |
Lease liability, non-current | | 275 | | | 350 | |
| Total operating lease liabilities | | $ | 801 | | | $ | 918 | |
The operating lease right-of-use (“ROU”) assets are included in property and equipment, net and the lease liabilities current and non-current are included in accrued expenses and other current liabilities and other long-term liabilities, respectively, in our unaudited condensed consolidated balance sheets. The Company utilized a weighted average discount rate of 9.6% to determine the present value of the lease payments.
The components of lease expense for the three months ended March 31, 2025 and 2024 were as follows (in thousands):
| | | | | | | | | | | | | | | | | | |
| | | Three Months Ended March 31, | | |
| | 2025 | | 2024 | | | | |
| Operating lease cost* | | $ | 146 | | | $ | 108 | | | | | |
*Includes short-term leases, which are immaterial.
The following table shows a maturity analysis of the operating lease liabilities as of March 31, 2025 (in thousands):
| | | | | | | | |
| | | |
| | | Undiscounted Cash Flows |
April 1, 2025 through December 31, 2025 | | $ | 420 | |
| 2026 | | 392 | |
| 2027 | | 63 | |
| 2028 | | — | |
| 2029 | | — | |
2030 | | — | |
| Thereafter | | — | |
| Total lease payments | | $ | 875 | |
| Less implied interest | | (74) | |
| Total | | $ | 801 | |
8. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities as of March 31, 2025 and December 31, 2024 consisted of the following (in thousands):
| | | | | | | | | | | | | | |
| | | As of |
| | | March 31, 2025 | | December 31, 2024 |
| Research and development | | $ | 1,177 | | | $ | 1,625 | |
| Compensation and benefits | | 1,770 | | | 2,883 | |
| General and administrative | | 415 | | | 380 | |
| | | | |
| | | | |
| Commercial operations | | 359 | | | 534 | |
| Royalty payment | | 327 | | | 327 | |
| Lease liability, current | | 526 | | | 568 | |
| | | | |
| Total accrued expenses and other current liabilities | | $ | 4,574 | | | $ | 6,317 | |
9. Capital Structure
Pursuant to the Company’s amended and restated certificate of incorporation, the Company is authorized to issue two classes of stock, common stock and preferred stock. At March 31, 2025, the total number of shares of capital stock the Company was authorized to issue was 205,000,000, of which 200,000,000 was common stock and 5,000,000 was preferred stock. All shares of common and preferred stock have a par value of $0.001 per share.
AlmataBio Transaction
On March 27, 2024, the Company acquired AlmataBio in which the former AlmataBio stockholders received (i) 171,605 shares of the Company’s common stock and (ii) 2,412 shares of the Company’s Series C Preferred Stock. Upon Company stockholder approval, which was obtained on August 13, 2024 and subject to beneficial ownership limitations, 2,063 shares of the Series C Preferred Stock issued to the former AlmataBio stockholders automatically converted into 2,062,930 shares of common stock. Refer to Note 3 - Asset Acquisition for more information regarding the acquisition and refer to sub-header “Series C Preferred Stock” within the “March 2024 Financing” section below for more information regarding the Series C Preferred Stock.
March 2024 Financing
On March 28, 2024, the Company closed a private placement investment in which the investors received (i) 19,946 shares of non-voting convertible Series C Preferred Stock, and (ii) warrants to purchase up to an aggregate of 11,967,526 shares of Avalo’s common stock (or a number of shares of Series C Preferred Stock convertible into the number of shares of common stock the warrant was then exercisable into), resulting in upfront gross proceeds of $115.6 million. Net proceeds were $108.1 million after deducting transaction costs of $7.5 million. The private placement transaction costs were expensed within other income (expense), net for the three months ended March 31, 2024. The Company received an additional $69.4 million of gross proceeds upon the full exercise of the warrants in the fourth quarter of 2024. Net proceeds were $67.6 million after deducting $1.7 million of transaction costs. Upon Company stockholder approval, which was obtained on August 13, 2024 and subject to beneficial ownership limitations, 6,585 shares of Series C Preferred Stock issued pursuant to the financing automatically converted into 6,585,314 shares of common stock. Additionally, the Company issued 781,259 shares of common stock and 11,186.267 shares of Series C Preferred Stock as a result of the warrant exercises in the fourth quarter of 2024.
Warrants on common stock or Series C Preferred Stock issued in March 2024 Financing
The warrants were exercisable via gross physical settlement for $5.796933 per underlying share of common stock (or a number of shares of Series C Preferred Stock convertible into the number of shares of common stock the warrant is then exercisable into). The warrants were fully exercised in the fourth quarter of 2024. The warrants included anti-dilution protection provisions.
The Company determined that the warrants did not satisfy the conditions to be accounted for as equity instruments. As the warrants did not meet the equity contract scope exception, the Company classified the warrants as a derivative liability upon issuance. The initial measurement of the warrants at fair value exceeded the proceeds received such that the difference between the initial fair value of the warrants and net upfront cash proceeds was recognized in the income statement as a loss. Subsequently, the warrants were carried at fair value with changes in fair value recognized in the Company’s unaudited condensed consolidated statements of operations and comprehensive loss until exercised. Upon exercise of the warrants in the fourth quarter of 2024, the warrant liability was valued at $73.3 million. The settlement of the $73.3 million warrant liability and related share issuance proceeds of $69.4 million resulted in a $142.7 million impact to the additional-paid-in-capital in the fourth quarter of 2024. The classification of the Series C preferred Stock in permanent equity is discussed below within the section “Series C Preferred Stock issued in the AlmataBio Transaction, March 2024 Financing and upon Warrant Exercises.”
The valuation of the warrants was considered under Level 3 of the fair value hierarchy due to the need to use assumptions in the valuation that are both significant to the fair value measurement and unobservable. Refer to Note 6 - Fair Value Measurements for additional information regarding the settlement and valuation of the warrant liability.
Series C Preferred Stock issued in the AlmataBio Transaction, March 2024 Financing and upon Warrant Exercises
As of March 31, 2025, the Company had 5,000,000 shares of Preferred Stock authorized, of which 34,326 have been designated as Series C Preferred Stock. As of March 31, 2025, there were 24,696 shares of series C Preferred Stock outstanding. The Series C Preferred Stock has a par value of $0.001 per share. The Series C Preferred Stock has no voting rights, no liquidation preference, and are not redeemable. In the event of any liquidation, dissolution or winding up of the Company, holders of Series C Preferred Stock are entitled to be paid out of the assets with the Company legally available for distribution to its stockholders on an as-converted and pari-passu basis with common stock. The Series C Preferred Stock is subject to broad-based weighted average anti-dilution protection for certain issuances of common stock and securities convertible into common stock. The Series C Preferred Stock is entitled to receive dividends equal to and in the same form, and in the same manner, based on the then-current conversion ratio as dividends actually paid on shares of the common stock, when, as and if such dividends are paid on shares of the common stock.
As a result of a contract amendment in the fourth quarter of 2024, the Series C Preferred Stock met equity classification and was recognized as a component of permanent stockholders’ equity within additional paid-in-capital. Prior to the contract amendment, the Series C Preferred Stock was contingently redeemable outside the control of the Company such that the Series C Preferred Stock was recognized outside of permanent equity. During the fourth quarter of 2024, the remaining 349 shares of Series C Preferred Stock held by the former AlmataBio stockholders, with a carrying value of $1.7 million, were reclassified to permanent equity. Additionally, the 11,186.267 shares of Series C Preferred Stock issued as a result of the warrant exercise in the fourth quarter of 2024, with a carrying value of $133.0 million, is recognized as a component of permanent stockholders’ equity within additional-paid-in capital on the Company’s unaudited condensed consolidated balance sheet.
No amounts were allocated to the Series C Preferred Stock issued pursuant to the March 2024 Financing because the initial fair value of the warrants exceeded gross proceeds received for the issuance of the private placement bundle that included both Series C Preferred Stock and warrants.
During the first quarter of 2025, 200 shares of Series C Preferred Stock were converted to 200,000 shares of common stock.
Series D and Series E Preferred Stock issued in the March 2024 Financing
As a condition to the March 2024 Financing, a single share of Series D Preferred Stock and a single Series E Preferred Stock were issued to two institutional investors that participated in the private placement. Both the Series D and the Series E Preferred Stock have a par value and liquidation preference of $0.001 per share. The Series D and Series E Preferred Stock do not have voting rights, are not entitled to dividends, and are not convertible into common stock. Each of the holders of the Series D and Series E Preferred Stock have the option to require the Company to redeem their shares at a price equal to the par value at any time. The Company retains the right to redeem the Series D and Series E Preferred Stock at a price equal to the par value if the holder owns less than a certain threshold of the Company’s outstanding common stock. Although the Series D and Series E Preferred Stock do not provide the holders with substantive economics, the Series D and Series E Preferred Stock were issued solely to allow for the institutional investors to appoint a director to the Company’s board of directors. Because the Series D and Series E Preferred Stock are redeemable at par value outside the control of the Company, they are recognized outside of permanent equity.
At-the-Market Offering Program
On May 4, 2023, the Company entered into an “at-the-market” sales agreement (the “Sales Agreement”) with Oppenheimer & Co. Inc. (“Oppenheimer”), pursuant to which the Company may sell, from time to time, shares of its common stock having an aggregate offering price of up to $9,032,567 through Oppenheimer. In August 2023, the Company and Oppenheimer entered into an amendment to the Sales Agreement (the “Amended Sales Agreement”) to increase the aggregate offering amount under the Sales Agreement to $50,000,000, inclusive of shares sold prior to the amendment. There were no sales under the Amended Sales Agreement program during the three months ended March 31, 2025 and 2024.
Common Stock Warrants
At March 31, 2025, the following common stock warrants were outstanding:
| | | | | | | | | | | | | | | | | |
| Number of common shares | | | Exercise price | | Expiration |
| underlying warrants | | | per share | | date |
| 148 | | | $ | 7,488.00 | | | June 2031 |
10. Stock-Based Compensation
2016 Equity Incentive Plan
In April 2016, our board of directors adopted the 2016 Equity Incentive Plan, which was approved by our stockholders in May 2016 and which was subsequently amended and restated in May 2018 and August 2019 with the approval of our board of directors and our stockholders. In June 2024, our board of directors approved a fourth amended and restated equity incentive plan, which was subsequently approved by the Company’s stockholders in August 2024 (the “2016 Fourth Amended Plan”). During the term of the 2016 Fourth Amended Plan, the share reserve will automatically increase on the first trading day in January of each calendar year ending on (and including) January 1, 2034, by an amount equal to 5% of the total number of outstanding shares of common stock and Series C Preferred Stock (determined on an as-converted stock basis) plus all outstanding prefunded warrants to acquire shares of common stock (if any) as of December 31 of the preceding calendar year. On January 1, 2025, pursuant to the terms of the 2016 Fourth Amended Plan, an additional 1,768,393 shares were made available for issuance. As of March 31, 2025, there were 1,387,443 shares available for future issuance under the 2016 Fourth Amended Plan.
Option grants expire after ten years. Employee options typically vest over four years. Employees typically receive a new hire option grant, as well as an annual grant in the first or second quarter of each year. Options granted to directors typically vest immediately or over a period of one or three years. Directors may elect to receive stock options in lieu of board compensation, which vest immediately. For stock options granted to employees and non-employee directors, the estimated grant date fair market value of the Company’s stock-based awards is amortized ratably over the individuals’ service periods, which is the period in which the awards vest. Stock-based compensation expense includes expense related to stock options, restricted stock units and employee stock purchase plan shares. The amount of stock-based compensation expense recognized for the three months ended March 31, 2025 and 2024 was as follows (in thousands):
| | | | | | | | | | | | | | | | | | |
| | | Three Months Ended March 31, | | |
| | | 2025 | | 2024 | | | | |
| Research and development | | $ | 1,301 | | | $ | 269 | | | | | |
| General and administrative | | 1,940 | | | 360 | | | | | |
| Total stock-based compensation | | $ | 3,241 | | | $ | 629 | | | | | |
Stock options with service-based vesting conditions
The Company has granted stock options that contain service-based vesting conditions. The compensation cost for these options is recognized on a straight-line basis over the vesting periods. A summary of option activity for the three months ended March 31, 2025 is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Options Outstanding |
| | | Number of shares | | Weighted average exercise price per share | | Weighted average grant date fair value per share | | Weighted average remaining contractual term (in years) |
| Balance at December 31, 2024 | | 1,999,749 | | | $ | 19.91 | | | $ | 14.98 | | | 9.6 |
| Granted | | 1,832,000 | | | $ | 7.92 | | | $ | 6.54 | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| Balance at March 31, 2025 | | 3,831,749 | | | $ | 14.18 | | | $ | 10.95 | | | 9.6 |
| | | | | | | | |
| Exercisable at March 31, 2025 | | 408,384 | | | $ | 53.59 | | | $ | 35.76 | | | 9.3 |
On January 1, 2025, the Company granted its newly appointed Chief Strategy Officer options with service-based vesting conditions to purchase 150,000 shares of common stock with an exercise price of $7.43 as an inducement option grant pursuant to Nasdaq Listing Rule 5635(c)(4). Additionally, on January 28, 2025, as part of its annual stock option award, the Company granted options with service-based vesting conditions to purchase 1.5 million shares of common stock to its employees that vest over four years.
The aggregate intrinsic value of stock options is calculated as the difference between the exercise price of the stock options and the fair value of the Company’s common stock for those stock options that had exercise prices lower than the fair value of the Company’s common stock. As of March 31, 2025, the aggregate intrinsic value of options outstanding was $0.2 million. There were 402,885 options that vested during the three months ended March 31, 2025 with a weighted average exercise price of $10.61 per share. The total grant date fair value of shares which vested during the three months ended March 31, 2025 and 2024 was $3.6 million and $0.6 million, respectively.
The Company recognized stock-based compensation expense of $2.4 million and $0.6 million related to stock options with service-based vesting conditions for the three months ended March 31, 2025 and 2024, respectively. At March 31, 2025, there was $26.0 million of total unrecognized compensation cost related to unvested service-based vesting condition awards. The unrecognized compensation cost is expected to be recognized over a weighted-average period of 3.2 years.
Stock-based compensation assumptions
The following table presents the assumptions used to compute stock-based compensation expense for stock options with service-based vesting conditions granted under the Black-Scholes valuation model for the three months ended March 31, 2025.
| | | | | | | | |
| Service-based options | | |
| Expected term of option (in years) | | 6.0 - 6.1 |
| Expected stock price volatility | | 99.9% - 104.1% |
| Risk-free interest rate | | 4.05% - 4.45% |
| Expected annual dividend yield | | 0% |
| |
Restricted Stock Units
The Company has granted RSUs that contain service-based vesting conditions. The Company measures the fair value of the RSUs using the stock price on the date of grant. The compensation cost for RSUs is recognized on a straight-line basis over the vesting period. There was no RSU activity for the three months ended March 31, 2024. A summary of RSU activity for the three months ended March 31, 2025 is as follows:
| | | | | | | | | | | | | | |
| | | RSUs Outstanding |
| | | Number of shares | | Weighted average grant date fair value |
Unvested RSUs at December 31, 2024 | | 632,100 | | | $ | 9.88 | |
| Granted | | — | | | — | |
| Vested | | (210,703) | | | $ | 9.88 | |
Unvested RSUs at March 31, 2025 | | 421,397 | | | |
The RSUs, which were granted on August 13, 2024, vest annually over a three-year period beginning on March 28, 2025. Accordingly, the first tranche of RSUs vested on March 28, 2025. The Company recognized stock-based compensation expense of $0.8 million related to RSUs for the three months ended March 31, 2025, and no stock-based compensation expense related to RSUs for the prior year period. At March 31, 2025, there was $4.1 million of total unrecognized compensation cost related to RSUs. The unrecognized compensation cost is expected to be recognized over a weighted-average period of 2.0 years.
Employee Stock Purchase Plan
On April 5, 2016, the Company’s board of directors approved the 2016 Employee Stock Purchase Plan, which was approved by the Company’s stockholders and became effective on May 18, 2016 (the “Initial ESPP”). In June 2024, our board of directors approved an amended and restated employee stock purchase plan, which was subsequently approved by the Company’s stockholders in August 2024 (the “ESPP”).
Under the ESPP, eligible employees can purchase common stock through accumulated payroll deductions at such times as are established by the administrator. The ESPP is administered by the compensation committee of the Company’s board of directors. Under the ESPP, eligible employees may purchase stock at 85% of the lower of the fair market value of a share of the Company’s common stock (i) on the first day of an offering period or (ii) on the purchase date. Eligible employees may contribute up to 15% of their earnings during the offering period. The Company’s board of directors may establish a maximum number of shares of the Company’s common stock that may be purchased by any participant, or all participants in the aggregate, during each offering period. Under the ESPP, a participant may not accrue rights to purchase more than $25,000 of the fair market value of the Company’s common stock for each calendar year in which such right is outstanding.
The Company initially reserved and authorized up to 174 shares of common stock for issuance under the Initial ESPP. Pursuant to the ESPP, on January 1 of each calendar year, the aggregate number of shares that may be issued under the ESPP automatically increases by a number equal to 1% of the Company’s outstanding shares of common stock and Series C Preferred Stock (determined on an as-converted basis) plus all outstanding prefunded warrants to acquire shares of common stock (if any), as of December 31 of the preceding calendar year. On January 1, 2025, the number of shares available for issuance under the ESPP increased by 353,679 shares. As of March 31, 2025, 580,256 shares remained available for issuance.
In accordance with the guidance in ASC 718-50, Employee Share Purchase Plans, the ability to purchase shares of the Company’s common stock at the lower of the offering date price or the purchase date price represents an option and, therefore, the ESPP is a compensatory plan under this guidance. Accordingly, stock-based compensation expense is determined based on the option’s grant-date fair value and is recognized over the requisite service period of the option. The Company used the Black-Scholes valuation model and recognized minimal stock-based compensation expense for the three months ended March 31, 2025 and 2024.
11. Income Taxes
The Company recognized minimal income tax expense for the three months ended March 31, 2025 and 2024 due to the significant valuation allowance against the Company’s deferred tax assets and the current and prior period losses.
12. Commitments and Contingencies
Litigation
Litigation - General
The Company may become party to various contractual disputes, litigation, and potential claims arising in the ordinary course of business. Reserves are established in connection with such matters when a loss is probable and the amount of such loss can be reasonably estimated. The Company currently does not believe that the resolution of such matters will have a material adverse effect on its financial position or results of operations except as otherwise disclosed in this report.
Possible Future Milestone Payments for In-Licensed Compounds
General
Avalo is a party to license and development agreements with various third parties, which contain future payment obligations such as royalties and milestone payments. The Company recognizes a liability (and related expense) for each milestone if and when such milestone is probable and can be reasonably estimated. As typical in the biotechnology industry, each milestone has unique risks that the Company evaluates when determining the probability of achieving each milestone and the probability of success evolves over time as the programs progress and additional information is obtained. The Company considers numerous factors when evaluating whether a given milestone is probable including (but not limited to) the regulatory pathway, development plan, ability to dedicate sufficient funding to reach a given milestone and the probability of success.
AVTX-009
On March 27, 2024, Avalo obtained the rights to an anti-IL-1β mAb (AVTX-009), including the world-wide exclusive license from Eli Lilly and Company (“Lilly”) (the “Lilly License Agreement”), pursuant to its acquisition of AlmataBio. AlmataBio had previously purchased the rights, title and interest in the asset from Leap Therapeutics, Inc. (“Leap”) in 2023, which have since been assumed by Avalo pursuant to its acquisition of AlmataBio (the “Leap Agreement”). Avalo is responsible for the development and commercialization of the program.
Avalo is required to pay up to $70.0 million based on the achievement of specified development and regulatory milestones to Lilly. Upon commercialization, the Company is required to pay sales-based milestones aggregating up to $650.0 million payable to Lilly and $70.0 million payable to Leap. There are no annual or maintenance fees payable under the Lilly License Agreement and Leap Agreement. Additionally, Avalo is required to pay royalties to Lilly during a country-by-country royalty term in which the low end and the high end of the range fall between 5% and 15% of Avalo or its sublicensees’ annual net sales. The royalty term due to Lilly commences on the date of first commercial sale of the licensed product in a given territory and expires on a county-by-country basis; on the latest of (a) the tenth (10th) anniversary of the date of the first commercial sale, (b) the expiration of the last-to-expire licensed patent in the given territory, or (c) the expiration of any data exclusivity period for the licensed product in the given territory.
The Lilly License Agreement remains in effect until the expiration of the last-to-expire royalty term of any licensed products. Each party may terminate for cause or by mutual agreement though the Company may terminate at its sole discretion by giving one-hundred twenty (120) days’ prior written notice to Lilly, in which case all licenses and rights granted pursuant to the agreement will automatically terminate and revert to Lilly. There are no termination or expiration provisions under the Leap Agreement.
Avalo has not paid any milestones, royalties or any other amounts under the Lilly License Agreement or Leap Agreement.
No expense related to the agreements was recognized in the three months ended March 31, 2025. There has been no cumulative expense recognized as of March 31, 2025 under the agreements. The Company will continue to monitor the milestones and royalties at each reporting period.
Refer to the sub-header below entitled “Acquisition Related and Other Contingent Liabilities” for information regarding future development milestones that are payable to the former AlmataBio stockholders.
Quisovalimab (AVTX-002)
KKC License Agreement
On March 25, 2021, the Company entered into a license agreement with Kyowa Kirin Co., Ltd. (“KKC”) for exclusive worldwide rights to develop, manufacture and commercialize quisovalimab, KKC’s first-in-class fully human anti-LIGHT (TNFSF14) monoclonal antibody for all indications (the “KKC License Agreement”). The KKC License Agreement replaced the Amended and Restated Clinical Development and Option Agreement between the Company and KKC dated May 28, 2020. Avalo is responsible for the development and commercialization of quisovalimab in all indications worldwide (other than the option in the KKC License Agreement that, upon exercise by KKC, allows KKC to develop, manufacture and commercialize quisovalimab in Japan). Avalo is not currently pursuing the clinical development of quisovalimab and is exploring strategic alternatives.
Under the KKC License Agreement, the Company paid KKC an upfront license fee of $10.0 million, which we recognized within research and development expenses in 2021. Avalo is also required to pay KKC up to an aggregate of $112.5 million based on the achievement of specified development and regulatory milestones. Upon commercialization, the Company is required to make milestone payments to KKC aggregating up to $75.0 million tied to the achievement of annual net sales targets. There are no annual or maintenance fees payable under the KKC License Agreement.
Additionally, the Company is required to pay KKC royalties during a country-by-country royalty term equal to a mid-teen percentage of annual net sales. The Company is required to pay KKC a mid-twenties percentage of the payments that the Company receives from sublicensing of its rights under the KKC License Agreement, subject to certain exclusions. The royalty term due to KKC commences on the date of first commercial sale of the licensed product in a given territory and expires on a county-by-country basis, on the latest of (a) the twelfth (12th) anniversary of the date of the first commercial sale, (b) the expiration of the last-to-expire licensed patent in the given territory, or (c) the expiration of any data exclusivity period for the licensed product in the given territory.
The KKC License Agreement remains in effect while the Company and its affiliates and sublicensees develop and commercialize quisovalimab subject to customary termination rights. Each party may terminate for cause though Avalo may terminate for convenience upon six (6) months’ prior written notice in the case where regulatory approval has not been obtained for the licensed product or upon twelve (12) months’ prior written notice where regulatory approval has been obtained for the licensed product.
As disclosed above, Avalo paid the $10.0 million upfront license fee in 2021. No further amounts have been paid related to the milestones, royalties or any other amounts under the agreement.
No expense related to the KKC License Agreement was recognized in the three months ended March 31, 2025. There has been no cumulative expense recognized as of March 31, 2025 related to the milestones, royalties or any other amounts other than the $10.0 million upfront license fee incurred in 2021 as disclosed above. The Company will continue to monitor the milestones and royalties at each reporting period.
CHOP License Agreement
Following its February 3, 2020 merger with Aevi Genomic Medicine, Inc. (“Aevi”), the Company became party to a license agreement with The Children’s Hospital of Philadelphia (“CHOP”) (as amended, the “CHOP License Agreement”). Quisovalimab became a covered product under this license agreement in 2021 and at that time became subject to the terms therein. Avalo is not currently pursuing the clinical development of quisovalimab and is exploring strategic alternatives.
An initial upfront fee of $0.5 million was paid to CHOP by Aevi, which Avalo acquired in 2020. Avalo is required to pay an additional $1.0 million to CHOP based on the achievement of specified regulatory and commercial milestones. Avalo is obligated to pay an annual license maintenance fee of $0.2 million to CHOP, of which Avalo has paid an aggregate of $1.1 million as of the filing date of this Quarterly Report on Form 10-Q.
The Company is also obligated to pay tiered royalties to CHOP on a country-to-country basis in which the low end and high end of the range are single-digit royalties based on the Company’s net sales of quisovalimab. The royalty term extends to the later of (a) fifteen years following the original date of the CHOP License Agreement, (b) the last-to-expire of the valid claims in the licensed patent rights covering the manufacture, sale, or use of quisovalimab and (c) the expiration of the regulatory exclusivity period for quisovalimab.
CHOP may terminate the CHOP License Agreement for the material default or insolvency of the Company, and the Company may terminate the CHOP License Agreement at will with six (6) months’ written notice.
As disclosed above, Aevi paid the $0.5 million upfront license fee and Avalo has paid $1.1 million of annual license fees. No further amounts have been paid related to the milestones, royalties or any other amounts under the agreement.
No expense related to the milestones and royalties due under the CHOP Agreement was recognized for the three months ended March 31, 2025. Avalo has not recognized any cumulative expense under the agreement related to the milestone or royalties as of March 31, 2025. The Company will continue to monitor the milestones and royalties at each reporting period.
AVTX-008 Sanford Burnham Prebys License Agreement
On June 21, 2021, the Company entered into an Exclusive Patent License Agreement with Sanford Burnham Prebys Medical Discovery Institute (the “Sanford Burnham Prebys License Agreement”) under which the Company obtained an exclusive license to a portfolio of issued patents and patent applications covering an immune checkpoint program (AVTX-008). Avalo is responsible for the development and commercialization of the program. Avalo is not currently pursuing the clinical development of AVTX-008 and is exploring strategic alternatives.
Under the terms of the Sanford Burnham Prebys License Agreement, the Company paid an upfront license fee of $0.4 million, as well as patent costs of $0.5 million, which we recognized within research and development expenses and within general and administrative expenses, respectively, in 2021. Additionally, Avalo pays a $40 thousand annual maintenance fee payable on the first anniversary of the effective date and each anniversary thereafter until the first commercial sale (of which Avalo has paid $0.1 million of annual maintenance fees as of the filing date of this Quarterly Report on Form 10-Q). The Company is required to pay Sanford Burnham Prebys up to approximately $24.2 million based on achievement of specified development and regulatory milestones. Upon commercialization, the Company is required to pay Sanford Burnham Prebys sales-based milestone payments aggregating up to $50.0 million tied to annual net sales targets.
Additionally, the Company is required to pay Sanford Burnham Prebys royalties during a country-by-country royalty term equal to a tiered low-to-mid single digit percentage of annual net sales. Avalo is also required to pay Sanford Burnham Prebys tiered payments in which the low end and high end of the range fall on or between 10% and 20% of what Avalo receives from sublicensing its rights under the Sanford Burnham Prebys License Agreement, subject to certain exclusions.
The Sanford Burnham Prebys License Agreement remains in effect until the expiration of the royalty term, which with respect to each product and country, continues until the expiration, invalidation or abandonment of the last of the licensed patent rights. Avalo may terminate the Sanford Burnham Prebys License Agreement at any time at its convenience upon providing at least ninety (90) days’ prior written notice. Sanford Burnham Medical Discovery Institute may terminate the Sanford Burnham Prebys License Agreement for cause.
As disclosed above, Avalo paid the $0.4 million upfront fee, as well as total patent costs of $0.5 million and $0.1 million of annual maintenance fees. No further amounts have been paid related to the milestones, royalties or any other amounts under the agreement.
No expense related to milestones or royalties pursuant to the Sanford Burnham Prebys License Agreement was recognized in the three months ended March 31, 2025. There has been no cumulative expense recognized as of March 31, 2025 related to the milestones or royalties under this license agreement other than the $0.4 million upfront fee incurred in 2021. The Company will continue to monitor the milestones and royalties at each reporting period.
AVTX-006 Astellas License Agreement
On July 15, 2019, the Company entered into an exclusive license agreement with OSI Pharmaceuticals, LLC, an indirect wholly owned subsidiary of Astellas Pharma, Inc. (“Astellas”), for the worldwide development and commercialization of the novel, second generation mTORC1/2 inhibitor (AVTX-006). Avalo is fully responsible for the development and commercialization of the program. Avalo is not currently pursuing the clinical development of AVTX-006 and is exploring strategic alternatives.
Under the terms of the license agreement, there was an upfront license fee of $0.5 million. The Company is required to pay Astellas up to an aggregate of $5.5 million based on the achievement of specified development and regulatory milestones. There are no annual maintenance fees payable under the Astellas license agreement. Additionally, the Company is required to pay Astellas a tiered mid-to-high single digit percentage of the payments that Avalo receives from any sublicensing of its rights under the Astellas license agreement, subject to certain exclusions. Upon commercialization, the Company is required to pay Astellas royalties during a country-by-country royalty term equal to a tiered mid-to-high single digit percentage of annual net sales during the period beginning upon the date of the first commercial sale of such licensed product in such country and ending on the later to occur of (a) the expiry of the last valid claim of an OSI product patent covering such licensed product in such country, (b) expiration of regulatory exclusivity in such country, and (c) ten (10) years from the first commercial sale of such licensed product in such country.
The Astellas License Agreement remains in effect on a country-by-country and licensed product-by-licensed product basis (in the territory), unless the license agreement is terminated earlier in accordance with the license agreement. Avalo may terminate the agreement at any time upon providing sixty (60) days’ written notice to Astellas and may terminate the agreement in its entirety without cause.
As disclosed above, Avalo paid the $0.5 million upfront license fee. No further amounts have been paid related to the milestones, royalties or any other amounts under the agreement.
No expense related to this license agreement was recognized in the three months ended March 31, 2025. There has been $0.5 million of cumulative expense recognized as of March 31, 2025 related to the milestones under this license agreement. The Company will continue to monitor the remaining milestones and royalties at each reporting period. The Company will continue to monitor the remaining milestones and royalties at each reporting period.
Possible Future Milestone Proceeds for Out-Licensed Compounds
AVTX-301 Out-License
On May 28, 2021, the Company out-licensed its rights in respect of its non-core asset, AVTX-301, to Alto Neuroscience, Inc. (“Alto”). The Company initially in-licensed the compound from an affiliate of Merck & Co., Inc. in 2013. Alto is fully responsible for the development and commercialization of the program.
Under the out-license agreement, the Company received a mid-six digit upfront payment from Alto, which we recognized as license revenue in 2021. The Company is also eligible to receive up to an aggregate of $18.6 million based on the achievement of specified development, regulatory and commercial sales milestones. Additionally, the Company is entitled to a less than single digit percentage royalty based on annual net sales.
The out-license agreement remains in effect on a licensed product-by-licensed product and country-by-country basis until the later of (i) the expiration of the last to expire valid patent claim covering such licensed product in such country, or (ii) 10 (ten) years after the first commercial sale of such licensed product in such country. Upon expiration of the agreement, the licenses shall become a fully paid-up, royalty-free, irrevocable, perpetual non-exclusive license and sublicense.
The Company had not recognized any milestones as of March 31, 2025 or received any payments other than the upfront payment as disclosed above.
AVTX-406 License Assignment
On June 9, 2021, the Company assigned its rights, title, interest, and obligations under an in-license covering its non-core asset, AVTX-406, to ES, a wholly owned subsidiary of Armistice, who was a significant stockholder of the Company at the time of the transaction and whose chief investment officer, Steven Boyd, and managing director, Keith Maher, served on Avalo’s Board until August 8, 2022. The transaction with ES was approved in accordance with Avalo’s related party transaction policy. ES is fully responsible for the development and commercialization of the program.
Under the assignment agreement, the Company received a low-six digit upfront payment from ES, which we recognized as license revenue in 2021. The Company is also eligible to receive up to an aggregate of $6.0 million based on the achievement of specified development and regulatory milestones. Upon commercialization, the Company is eligible to receive sales-based milestone payments aggregating up to $20.0 million tied to annual net sales targets.
The Company had not recognized any milestones as of March 31, 2025 or received any payments other than the upfront payment as disclosed above.
AVTX-800 Series Asset Sale
On October 27, 2023, the Company sold its rights, title and interests in AVTX-801, AVTX-802 and AVTX-803 (collectively, the “800 Series”) to AUG Therapeutics, LLC (“AUG”). AUG is fully responsible for the development and commercialization of the program.
Pursuant to the Purchase Agreement with AUG, the Company received an upfront payment of $0.2 million. Additionally, AUG assumed aggregate liabilities of $0.4 million, which included certain liabilities incurred prior to the date of the Purchase Agreement, costs due and payable between the date of the Purchase Agreement and the closing date, and obligations under 800 Series contracts assumed by AUG. Avalo is also entitled to a contingent milestone payment of 20% of certain amounts, if any, granted to AUG upon sale of any priority review voucher related to the 800 Series compounds granted to AUG by the FDA, net of any selling costs, or $15.0 million for each compound (for a potential aggregate of $45.0 million) if the first FDA approval is for any indication other than a Rare Pediatric Disease (as defined in the Purchase Agreement).
The Company had not recognized any revenue related to the milestones as of March 31, 2025 or received any payments other than the upfront payment and reimbursement for certain liabilities as disclosed above.
Acquisition Related and Other Contingent Liabilities
AlmataBio Transaction Possible Future Milestone Payments
On March 27, 2024, the Company acquired AVTX-009 through its acquisition of AlmataBio. Pursuant to the AlmataBio Transaction, the Company made a cash payment of $7.5 million in April 2024 to the former AlmataBio stockholders, which was due upon the initial closing of the private placement on March 28, 2024 (the “Initial Milestone”). Further, a portion of the consideration for the AlmataBio transaction includes development milestones to the former AlmataBio stockholders including $5.0 million due upon the first patient dosed in a Phase 2 trial in patients with hidradenitis suppurativa for AVTX-009 (the “Second Milestone”), which was met and paid in October 2024 as discussed below, and $15.0 million due upon the first patient dosed in a Phase 3 trial for AVTX-009 (the “Third Milestone”), both of which are payable in cash or stock of Avalo at the election of the former AlmataBio stockholders. In the absence of timely notice of such election, Avalo may elect to pay the milestones in cash or common stock of Avalo.
The Company paid the Initial Milestone payment in April 2024 and recognized the payment within acquired in-process research and development expense. In addition, the Company concluded the Second Milestone was probable as of the acquisition date and therefore recognized the $5.0 million milestone within acquired in-process research and development expense in the condensed consolidated statements of operations and comprehensive income (loss) for the three months ended March 31, 2024 and the corresponding liability as contingent consideration as of March 31, 2024. The Company made a cash payment of $5.0 million in October 2024 upon meeting the Second Milestone. The Company will continue to monitor the Third Milestone each reporting period.
AVTX-006 Royalty Agreement with Certain Related Parties
In July 2019, Aevi entered into a royalty agreement, and liabilities thereunder were assumed by Avalo upon close of the Aevi Merger in February 2020. The royalty agreement provided certain investors, including LeoGroup Private Investment Access, LLC on behalf of Garry Neil, the Company’s Chief Executive Officer and Chairman of the Board, and Mike Cola, the Company’s former Chief Executive Officer (collectively, the “Investors”), a royalty stream, in exchange for a one-time aggregate payment of $2.0 million (the “Royalty Agreement”). Pursuant to the Royalty Agreement, the Investors will be entitled collectively to an aggregate amount equal to a low-single digit percentage of the aggregate net sales of the Company’s second generation mTORC1/2 inhibitor, AVTX-006 for a royalty term consistent with the royalty term disclosed in the AVTX-006 Astellas License Agreement section above. Avalo considers AVTX-006 a non-core asset and is exploring strategic alternatives. At any time beginning three years after the date of the first public launch of AVTX-006, Avalo may exercise, at its sole discretion, a buyout option that terminates any further obligations under the Royalty Agreement in exchange for a payment to the Investors of an aggregate of 75% of the net present value of the royalty payments. A majority of the independent members of the board of directors and the audit committee of Aevi approved the Royalty Agreement.
Avalo assumed this Royalty Agreement upon closing of the Aevi Merger and it is recorded as a royalty obligation within the Company's accompanying unaudited condensed consolidated balance sheet as of March 31, 2025 and December 31, 2024. Because there is a significant related party relationship between the Company and the Investors, the Company has treated its obligation to make royalty payments under the Royalty Agreement as an implicit obligation to repay the funds advanced by the Investors. As the Company makes royalty payments in accordance with the Royalty Agreement, it will reduce the liability balance. At the time that such royalty payments become probable and estimable, and if such amounts exceed the liability balance, the Company will impute interest accordingly on a prospective basis based on such estimates, which will result in a corresponding increase in the liability balance.
Karbinal Royalty Make-Whole Provision
In 2018, in connection with the acquisition of certain commercialized products, the Company entered into a supply and distribution agreement (the “Karbinal Agreement”) with TRIS Pharma Inc. (“TRIS”). As part of the Karbinal Agreement, the Company had an annual minimum sales commitment, which is based on a commercial year that spans from August 1 through July 31, of 70,000 units through 2025. The Company was required to pay TRIS a royalty make whole payment (“Make-Whole Payments”) of $30 for each unit under the 70,000 units annual minimum sales commitment through 2025.
As a part of the Aytu Transaction, the Company assigned all its payment obligations, including the Make-Whole Payments, under the Karbinal Agreement (collectively, the “TRIS Obligations”) to Aytu. However, under the original license agreement, the Company could ultimately be liable for the TRIS Obligations to the extent Aytu fails to make the required payments. The future Make-Whole Payments to be made by Aytu are unknown as the amount owed to TRIS is dependent on the number of units sold.
13. Segments
The Company’s chief operating decision maker (“CODM”), who is our Chief Executive Officer, views the Company’s operations and manages the business as one operating segment. The presentation of financial results as one reportable segment is consistent with the way we operate our business and the manner in which our CODM evaluates performance and makes resource and operating decisions for the business. The accounting policies of the business segment are the same as those described in the summary of significant accounting policies as contained in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024.
The CODM evaluates performance and makes resource and operating decisions for the business based on net loss as reported on the unaudited condensed consolidated statement of operations and total assets as reported on the unaudited condensed consolidated balance sheet. The CODM’s primary evaluation of the Company’s success is its ability to progress its research and development pipeline programs toward commercialization or opportunistically out-license rights to indications or geographies. The CODM uses net loss compared to budget and/or forecast amounts to evaluate this progress to make resource and operating decisions, such as whether to issue equity and/or make new investments in additional indications or pipeline assets. Additionally, the Company’s CODM periodically reviews research and development expense, as stated on the condensed consolidated statement of operations, and treats it as a significant segment expense. The CODM considers research and development expense in the context of achieving the next expected milestone in the pipeline, and will make resource and operating decisions accordingly, such as decisions on raising additional capital and/or pursuing additional indications or programs. The following table summarizes our research and development expenses for the three months ended March 31, 2025 and 2024:
| | | | | | | | | | | | | | |
| | Three Months Ended March 31, |
| | 2025 | | 2024 |
| Nonclinical expenses | | $ | 83 | | | $ | 152 | |
| Clinical expenses | | 3,850 | | | 62 | |
| CMC expenses | | 1,979 | | | 254 | |
| | | | |
| Internal expenses: | | | | |
| Salaries, benefits and related costs | | 1,850 | | | 1,324 | |
| Stock-based compensation expense | | 1,301 | | | 269 | |
| Other | | 60 | | | 55 | |
| | $ | 9,123 | | | $ | 2,116 | |
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Quarterly Report on Form 10-Q and the information incorporated herein by reference contain forward-looking statements that involve a number of risks and uncertainties, as well as assumptions that, if they never materialize or prove incorrect, could cause our results to differ materially from those expressed or implied by such forward-looking statements. Forward-looking statements can be identified by the use of forward-looking words such as “projects,” “may,” “might,” “will,” “could,” “would,” “should,” “continue,” “seeks,” “aims,” “predicts,” “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “potential,” “pro forma” or other similar words (including their use in the negative), or by discussions of future matters such as: the future financial and operational outlook; the development of product candidates; and other statements that are not historical. Although our forward-looking statements reflect the good faith judgment of our management, these statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties, and actual results and outcomes may differ materially from results and outcomes discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those set out in our Annual Report on Form 10-K filed with the SEC on March 20, 2025, and in our other filings with the SEC. Statements made herein are as of the date of the filing of this Quarterly Report on Form 10-Q with the SEC and should not be relied upon as of any subsequent date. Unless otherwise required by applicable law, we do not undertake, and we specifically disclaim any obligation to update any forward-looking statements to reflect occurrences, developments, unanticipated events or circumstances after the date of such statement.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our unaudited financial statements and related notes that appear in Item 1 of this Quarterly Report on Form 10-Q and with our audited financial statements and related notes for the year ended December 31, 2024 appearing in our Annual Report on Form 10-K filed with the SEC on March 20, 2025.
Overview
Avalo Therapeutics, Inc. (the “Company,” “Avalo” or “we”) is a clinical stage biotechnology company focused on the treatment of immune dysregulation. Avalo’s lead asset is AVTX-009, an anti-IL-1β monoclonal antibody (“mAb”), targeting inflammatory diseases.
Our focus in 2025 is continuing to execute operationally on the development of AVTX-009, most notably the progression of the LOTUS trial.
Management’s primary evaluation of the success of the Company is the ability to progress its pipeline forward toward commercialization or opportunistically out-licensing rights to indications or geographies. We believe the ability to achieve the anticipated milestone as presented in the following chart represents our most immediate evaluation point as to the progress of our goal to move the pipeline forward.
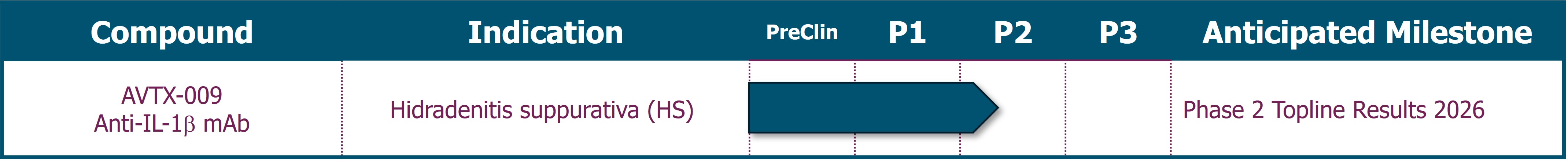
The Company’s Phase 2 (“LOTUS”) trial of AVTX-009, an anti-IL-1β (mAb), in hidradenitis suppurativa (“HS”), is a randomized, double-blind, placebo-controlled, parallel-group Phase 2 trial with two AVTX-009 dose regimens to evaluate the efficacy, safety and tolerability of AVTX-009 in approximately 180 adults with moderate to severe HS. Subjects will be randomized (1:1:1) to receive either one of two doses of AVTX-009 or placebo. The primary efficacy endpoint is the proportion of subjects achieving Hidradenitis Suppurativa Clinical Response (HiSCR75) at Week 16. Avalo is the study sponsor and the current proposed trial locations include the United States, Canada, France, Germany, Italy, Spain, Bulgaria, Czech Republic, Greece, Poland, Australia and Turkey.
Recent Developments
In March 2025, the Company announced the appointment of Michael Heffernan as Chairman of the Board of Directors. Mr. Heffernan’s extensive experience in building and leading biopharmaceutical companies and driving shareholder value combined with his leadership skills and board experience makes him a valuable member of our Board of Directors.
Liquidity
Since inception, we have incurred significant operating and cash losses from operations. We have primarily funded our operations to date through sales of equity securities, out-licensing transactions and sales of assets.
For the three months ended March 31, 2025, Avalo generated a net loss of $13.1 million and negative cash flows from operations of $9.5 million. As of March 31, 2025, Avalo had $125.0 million in cash and cash equivalents.
Based on our current operating plans, we expect that our existing cash and cash equivalents are sufficient to fund operations for at least twelve months from the filing date of this Quarterly Report on Form 10-Q and we expect current cash on hand to fund operations into at least 2027. The Company closely monitors its cash and cash equivalents and seeks to balance the level of cash and cash equivalents with our projected needs to allow us to withstand periods of uncertainty relative to the availability of funding on favorable terms. We may satisfy any future cash needs through sales of equity securities under the Company’s at-the-market program or other equity financings, out-licensing transactions, strategic alliances/collaborations, sale of programs, and/or mergers and acquisitions. There can be no assurance that any financing or business development initiatives can be realized by the Company, or if realized, what the terms may be. To the extent that we raise capital through the sale of equity, the ownership interest of our existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our stockholders. Further, if the Company raises additional funds through collaborations, strategic alliances or licensing arrangements with third parties, the Company might have to relinquish valuable rights to its technologies, future revenue streams, research programs or product candidates.
Our Strategy
Our strategy for increasing stockholder value includes:
•Advancing our pipeline through development to regulatory approval. Most notably and in the near term, completing our Phase 2 LOTUS trial in hidradenitis suppurativa, preparing for the next stage of development for that indication and considering further indication expansion for AVTX-009;
•Acquiring or in-licensing rights to and/or developing targeted, complementary differentiated preclinical and clinical stage compounds that treat immune mediated disease; and
•Opportunistically out-licensing rights to compounds, indications or geographies.
There is no guarantee that our products will obtain regulatory approval by the United States Food and Drug Administration (the “FDA”) or comparable foreign regulatory authorities. The FDA approval process is complex, time-consuming, and expensive. It typically involves the following prior to submitting a new drug application (“NDA”) or biologics license application (“BLA”): preclinical laboratory and animal testing, submission of an Investigational New Drug (“IND”) application, and human clinical trials to establish safety and efficacy. Human clinical trials typically include: Phase 1 studies to evaluate the safety and tolerability of the drug, generally in normal, healthy volunteers; Phase 2 studies to evaluate safety and efficacy, as well as appropriate doses; these studies are typically conducted in patient volunteers who suffer from the particular disease condition that the drug is designed to treat; and Phase 3 studies to evaluate safety and efficacy of the product at specific doses in one or more larger pivotal trials. Upon submission of an NDA or BLA, the FDA reviews the application including potentially an FDA advisory committee review and typically inspects manufacturing facilities and clinical study sites prior to FDA approval or rejection of the application. Even if a product receives FDA approval, the agency may impose post-approval requirements or withdraw approval if safety or efficacy issues arise. The processes for obtaining marketing approvals in foreign countries, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources.
Results of Operations
Comparison of the Three Months Ended March 31, 2025 and 2024
Cost of Product Sales
We recognized no cost of product sales for the three months ended March 31, 2025, compared to minimal activity for the same period in 2024.
The Company will continue to monitor estimates for commercial liabilities, such as sales returns, profit share with the supplier pursuant to the reconciliation process, and commercial activity with Aytu, who previously managed Millipred® commercial operations on our behalf. As additional information becomes available, the Company could recognize expense (or a benefit) for differences between actuals or updated estimates to the reserves previously recognized, which could be recognized in cost of product sales.
Research and Development Expenses
The following table summarizes our research and development expenses for the three months ended March 31, 2025 and 2024 (in thousands):
| | | | | | | | | | | | | | |
| | | Three Months Ended March 31, |
| | | 2025 | | 2024 |
| Nonclinical expenses | | $ | 83 | | | $ | 152 | |
| Clinical expenses | | 3,850 | | | 62 | |
| CMC expenses | | 1,979 | | | 254 | |
| | | | |
| Internal expenses: | | | | |
| Salaries, benefits and related costs | | 1,850 | | | 1,324 | |
| Stock-based compensation expense | | 1,301 | | | 269 | |
| Other | | 60 | | | 55 | |
| | | $ | 9,123 | | | $ | 2,116 | |
Research and development expenses increased $7.0 million for the three months ended March 31, 2025 compared to the three months ended March 31, 2024. This increase was mainly driven by a $3.8 million increase in clinical expenses, $1.7 million increase in chemistry, manufacturing, and controls (“CMC”) expenses, and $1.0 million increase in stock-based compensation expense.
AVTX-009 was acquired in late March 2024 as part of the AlmataBio Transaction (as defined in Note 3 to the unaudited condensed consolidated financial statements). As such, both clinical expenses and CMC expenses increased due to the ongoing LOTUS trial activities in the first quarter of 2025, compared to no expenses related to the trial for the same period in the prior year.
Stock-based compensation increased $1.0 million compared to the three months ended March 31, 2024 due to option and restricted stock unit grants made during the period, including the annual employee grants in August 2024 and January 2025, as well as headcount additions.
We expect future research and development expenses to increase as a result of our development plans for AVTX-009, including the execution of the Phase 2 LOTUS trial which commenced in October 2024.
General and Administrative Expenses
The following table summarizes our general and administrative expenses for the three months ended March 31, 2025 and 2024 (in thousands):
| | | | | | | | | | | | | | |
| | | Three Months Ended March 31, |
| | | 2025 | | 2024 |
| | | | |
| Salaries, benefits and related costs | | $ | 1,553 | | | $ | 902 | |
| Legal, consulting and other professional expenses | | 1,611 | | | 1,590 | |
| Stock-based compensation expense | | 1,940 | | | 360 | |
Commercial planning and marketing expenses | | 12 | | | 7 | |
| Other | | 430 | | | 334 | |
| | | $ | 5,546 | | | $ | 3,193 | |
General and administrative expenses increased $2.4 million for the three months ended March 31, 2025 compared to the prior period. The increase was driven by a $1.6 million increase in stock-based compensation expense due to option and restricted stock unit grants made during the period, including the annual grants in August 2024 and January 2025, as well as headcount additions.
While we expect the majority of operating expense increases will be focused on research and development activities to progress AVTX-009, we also expect moderate increases to general and administrative expenses to support the AVTX-009 program.
Other Income (Expense), Net
The following table summarizes our other income (expense), net for the three months ended March 31, 2025 and 2024 (in thousands):
| | | | | | | | | | | | | | |
| | | Three Months Ended March 31, |
| | | 2025 | | 2024 |
| Change in fair value of derivative liability | | 380 | | | (120) | |
Interest income, net | | 1,148 | | | 100 | |
| Excess of initial warrant fair value over private placement proceeds | | — | | | (79,276) | |
| Private placement transaction costs | | — | | | (9,220) | |
| | | | |
| | | | |
| | $ | 1,528 | | | $ | (88,516) | |
Other income, net increased $90.0 million for the three months ended March 31, 2025 compared to the prior period. The increase was primarily due to the excess of the warrant fair value over proceeds received from the March 2024 private placement financing that occurred during the three months ended March 31, 2024. The warrants did not meet the equity contract scope exception and therefore were classified as a liability upon issuance. The initial measurement of the warrant liability of $194.9 million exceeded the proceeds received of the private placement investment of $115.6 million, which resulted in a $79.3 million loss recognized in other expense, net. The warrants were fully exercised in the fourth quarter of 2024. Refer to Note 6 - Fair Value Measurements of the unaudited condensed consolidated financial statements for more information. Similarly, in the first quarter of 2024, we recognized $9.2 million of private placement transaction cost associated with the March 2024 financing that did not repeat in the current period. Finally, interest income increased from the prior period driven by the increased cash balance in the current year compared to the prior year.
Income Tax Expense
The Company recognized minimal income tax expense for both the three months ended March 31, 2025 and 2024.
Liquidity and Capital Resources
Uses of Liquidity
The Company primarily uses cash to fund the ongoing development of our research and development pipeline assets, mainly AVTX-009, and costs associated with its organizational infrastructure. As of March 31, 2025, Avalo had $125.0 million in cash and cash equivalents.
Cash Flows
The following table summarizes our cash flows for the three months ended March 31, 2025 and 2024 (in thousands):
| | | | | | | | | | | | | | |
| | | Three Months Ended March 31, |
| | | 2025 | | 2024 |
| | | | |
| Net cash (used in) provided by: | | | | |
| Operating activities | | $ | (9,457) | | | $ | (6,202) | |
| Investing activities | | — | | | 356 | |
| Financing activities | | — | | | 108,612 | |
Net (decrease) increase in cash and cash equivalents | | $ | (9,457) | | | $ | 102,766 | |
Net cash used in operating activities
Net cash used in operating activities was $9.5 million for the three months ended March 31, 2025 and consisted primarily of net loss of $13.1 million and adjustments to reconcile net loss to net cash used in operating activities including stock-based compensation of $3.2 million. Prepaid expense decreased $2.5 million primarily due to the timing of AVTX-009 related payments. Accrued expenses and other liabilities decreased $1.7 million primarily related to the timing of non-equity incentive compensation.
Net cash used in operating activities was $6.2 million for the three months ended March 31, 2024 and consisted primarily of a net loss of $121.3 million and adjustments to reconcile net loss to net cash used in operating activities including the excess of warrant fair value over private placement investment proceeds of $79.3 million, acquired IPR&D of $27.5 million, transaction costs payable upon exercise of the warrants issued pursuant to the private placement investment of $1.7 million, and stock-based compensation of $0.6 million. Accrued expenses and other liabilities increased primarily due to the $1.7 million transaction costs payable upon exercise of the warrants issued pursuant to the private placement.
We expect future cash used in operating activities to increase in future periods as a result of our development plans for AVTX-009, including the execution of the Phase 2 LOTUS trial, which commenced in October 2024.
Net cash provided by investing activities
There was no net cash provided by investing activities for the three months ended March 31, 2025.
Net cash used in investing activities was for the three months ended March 31, 2024 consisted of the cash acquired as part of the AlmataBio Transaction.
Net cash provided by financing activities
There was no net cash provided by financing activities for the three months ended March 31, 2025.
Net cash provided by financing activities for the three months ended March 31, 2024 consisted of gross proceeds of $115.6 million from the private placement investment that closed on March 28, 2024, partially offset by transaction costs related to the private placement investment of $7.0 million.
Critical Accounting Policies, Estimates, and Assumptions
This Management’s Discussion and Analysis of Financial Condition and Results of Operations is based on our unaudited condensed consolidated financial statements included in this Quarterly Report on Form 10-Q, which have been prepared in accordance with GAAP. In preparing the financial statements in conformity with GAAP, the Company makes estimates and assumptions that have an impact on assets, liabilities, revenue and expenses reported. These estimates can also affect supplemental information disclosed by us, including information about contingencies, risk, and financial condition. In our unaudited condensed consolidated financial statements, estimates are used for, but not limited to, revenue recognition, cost of product sales, stock-based compensation, fair value measurements, the valuation of derivative liabilities, cash flows used in management’s going concern assessment, income taxes, goodwill, and clinical trial accruals. The Company believes, given current facts and circumstances, that our estimates and assumptions are reasonable, adhere to GAAP and are consistently applied. Inherent in the nature of an estimate or assumption is the fact that actual results may differ from estimates, and estimates may vary as new facts and circumstances arise. Our most critical accounting estimates and assumptions are included in our Annual Report on Form 10-K for the year ended December 31, 2024 filed with the SEC on March 20, 2025. There have been no significant changes to our critical accounting policies during the three months ended March 31, 2025.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, as defined by applicable SEC rules and regulations.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
As a smaller reporting company, we are not required to provide the information required by this Item.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
As required by Rule 13a-15(b) and Rule 15d-15(b) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, our management, including our principal executive officer and our principal financial officer, conducted an evaluation as of the end of the period covered by this Quarterly Report on Form 10-Q of the effectiveness of the design and operation of our disclosure controls and procedures. In designing and evaluating our disclosure controls and procedures, our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of the end of the period covered by this Quarterly Report on Form 10-Q.
Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during the period covered by this Quarterly Report on Form 10-Q that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II – OTHER INFORMATION
Item 1. Legal Proceedings.
The information set forth in Note 12 - Commitments and Contingencies, under the heading “Litigation” to our unaudited condensed consolidated financial statements, included in Part I, Item 1 of this Quarterly Report on Form 10-Q, is incorporated herein by reference.
Item 1A. Risk Factors.
In addition to the other information set forth in this Quarterly Report on Form 10-Q, you should carefully consider the factors discussed in Part I, “Item 1A. Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on March 20, 2025 (the “2024 10-K”), which could materially affect our business, financial condition, or future results. Our risk factors as of the date of this Quarterly Report on Form 10-Q have not changed materially from those described in the 2024 10-K referenced above. The risks described in the 2024 10-K referenced above, however, are not the only risks facing our Company. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially adversely affect our business, financial condition, or future results of operations and the trading price of our common stock.
Item 6. Exhibits.
| | | | | | | | |
Exhibit
Number | | Description of Exhibit |
| | |
| 10.1 | | |
| | |
10.2* | | |
| | |
| 31.1+ | | |
| | |
| 31.2+ | | |
| | |
| 32.1† | | |
| | |
| 101 | | Interactive data files pursuant to Rule 405 of Regulation S-T: (i) Condensed Consolidated Balance Sheets as of March 31, 2025 (Unaudited) and December 31, 2024; (ii) Condensed Consolidated Statements of Operations and Comprehensive Loss (Unaudited) for the Three Months Ended March 31, 2025 and 2024; (iii) Condensed Consolidated Statements of Cash Flows (Unaudited) for the Three Months Ended March 31, 2025 and 2024; (iv) Condensed Consolidated Statements of Mezzanine and Stockholders’ Equity (Unaudited) for the Three Months Ended March 31, 2025 and 2024; and (v) Notes to Unaudited Financial Statements. |
| | |
| 104 | | Cover Page Interactive Data File, formatted in XBRL (included in Exhibit 101). |
| | |
* Management contract or compensatory arrangement.
+ Filed herewith.
† This certification is being furnished solely to accompany this Quarterly Report on Form 10-Q pursuant to 18 U.S.C. Section 1350, and is not being filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing of the registrant, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
| | | | | | | | | | | | | | |
| SIGNATURES | |
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized. | |
| | | | |
| | | Avalo Therapeutics, Inc. | |
| | | | |
Date: May 12, 2025 | | /s/ Christopher Sullivan | |
| | | Christopher Sullivan | |
| | | Chief Financial Officer | |
| | | (on behalf of the registrant and as the registrant’s principal financial officer) | |
| | | |

