
Exhibit 99.1

1 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Innovation Driven by Compassion September 2021

2 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. This presentation may include forward - looking statements made pursuant to the Private Securities Litigation Reform Act of 1995. Forward - looking statements are statements that are not historical facts. Such forward - looking statements are subject to signific ant risks and uncertainties that are subject to change based on various factors (many of which are beyond the control of Avalo Therapeu tic s, Inc. (“Avalo” or the “Company”)), which could cause actual results to differ from the forward - looking statements. Such statements may include, without limitation, statements with respect to Avalo’s plans, objectives, projections, expectations and intentions a nd other statements identified by words such as “projects,” “may,” “might,” “will,” “could,” “would,” “should,” “continue,” “seeks,” “ aim s,” “predicts,” “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “potential,” or similar expressions (inclu din g their use in the negative), or by discussions of future matters such as: its future financial and operational outlook; the development of pro duct candidates or products; potential attributes and benefits of product candidates; strategic alternatives for Millipred ; and other statements that are not historical. These statements are based upon the current beliefs and expectations of Avalo’s management but are subject to significant ris ks and uncertainties, including: reliance on and integration of key personnel; drug development costs, timing and other risks, inclu din g reliance on investigators and enrollment of subjects in clinical trials, which might be slowed by the COVID - 19 pandemic; regulatory risks; Avalo’s cash position and the need for it to raise additional capital; risks related to potential strategic alternatives for Millipred ; general economic and market risks and uncertainties, including those caused by the COVID - 19 pandemic and those other risks detailed in A valo’s filings with the Securities and Exchange Commission. Actual results may differ from those set forth in the forward - looking state ments. Except as required by applicable law, Avalo expressly disclaims any obligations or undertaking to release publicly any update s o r revisions to any forward - looking statements contained herein to reflect any change in Avalo’s expectations with respect thereto or any change in events, conditions, or circumstances on which any statement is based. Forward - Looking Statements

3 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • Avalo has a pipeline of six novel, first - in - class assets in eight clinical development programs across Immunology, Immuno - oncology, and Rare Genetic Diseases • All assets have demonstrated mechanistic rationale, biomarkers, or established proof - of - concept (POC) to increase probability of success • AVTX - 002 (anti - LIGHT mAb*) demonstrated clinically meaningful endoscopic improvement in 75% (3/4) of subjects in the initial results (Cohort 1) of a Phase 1b moderate to severe Crohn’s disease clinical trial • AVTX - 002 demonstrated statistically significant improvement in the primary endpoint of alive and free of respiratory failure status in a Phase 2 COVID - 19 ARDS clinical trial • Currently, four assets have been designated ODD* and RPDD* enabling Priority Review Vouchers (would provide non - dilutive financing of the pipeline) • Multiple data readouts anticipated in the second half of 2021 Pipeline Highlights *Orphan Drug Designation, Rare Pediatric Disease Designation; eligibility for Priority Review Voucher Upon Approval. mAb, monoclonal antibody.
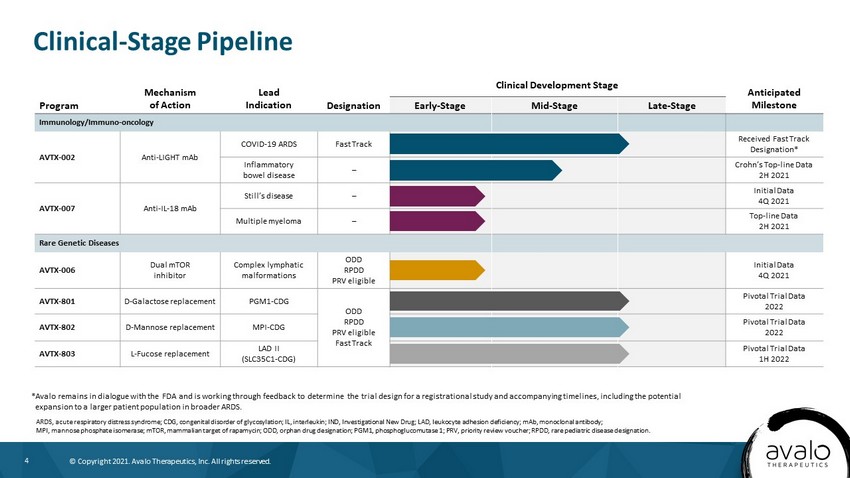
4 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Clinical - Stage Pipeline *Avalo remains in dialogue with the FDA and is working through feedback to determine the trial design for a registrational st udy and accompanying timelines, including the potential expansion to a larger patient population in broader ARDS. ARDS, acute respiratory distress syndrome; CDG, congenital disorder of glycosylation; IL, interleukin; IND, Investigational N ew Drug; LAD, leukocyte adhesion deficiency; mAb, monoclonal antibody; MPI, mannose phosphate isomerase; mTOR, mammalian target of rapamycin; ODD, orphan drug designation; PGM1, phosphoglucomutase 1; PRV, priority review voucher; RPDD, rare pediatric disease designation. Program Mechanism of Action Lead Indication Designation Clinical Development Stage Anticipated Milestone Early - Stage Mid - Stage Late - Stage Immunology/Immuno - oncology AVTX - 002 Anti - LIGHT mAb COVID - 19 ARDS Fast Track Received Fast Track Designation* Inflammatory bowel disease – Crohn’s Top - line Data 2H 2021 AVTX - 007 Anti - IL - 18 mAb Still’s disease – Initial Data 4Q 2021 Multiple myeloma – Top - line Data 2H 2021 Rare Genetic Diseases AVTX - 006 Dual mTOR inhibitor Complex lymphatic malformations ODD RPDD PRV eligible Initial Data 4Q 2021 AVTX - 801 D - Galactose replacement PGM1 - CDG ODD RPDD PRV eligible Fast Track Pivotal Trial Data 2022 AVTX - 802 D - Mannose replacement MPI - CDG Pivotal Trial Data 2022 AVTX - 803 L - Fucose replacement LAD II (SLC35C1 - CDG) Pivotal Trial Data 1H 2022

5 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. 5 Anti - LIGHT monoclonal antibody in clinical studies for Crohn’s Disease and COVID - 19 ARDS AVTX - 002
6 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • Novel, first - in - class fully human subcutaneous (SQ) monoclonal antibody (mAb) • Only fully human anti - LIGHT mAb • Only anti - LIGHT mAb in clinical development AVTX - 002: A Novel First - in - Class Anti - LIGHT (TNFSF14) mAb *Kyowa Kirin has an option to retain the rights in Japan. In - licensed From Kyowa Kirin Co., Worldwide Exclusive Rights* for All Indications (2021)

7 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. 7 AVTX - 002: Crohn’s Disease
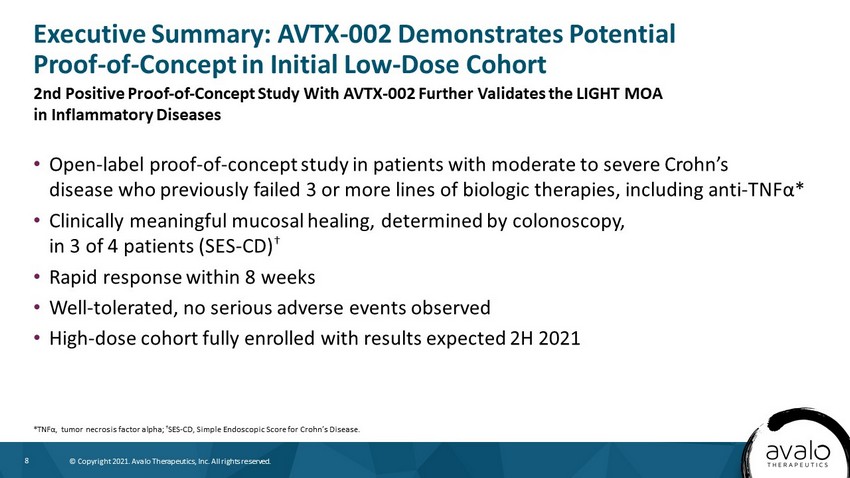
8 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • Open - label proof - of - concept study in patients with moderate to severe Crohn’s disease who previously failed 3 or more lines of biologic therapies, including anti - TNF α * • Clinically meaningful mucosal healing, determined by colonoscopy, in 3 of 4 patients (SES - CD) † • Rapid response within 8 weeks • Well - tolerated, no serious adverse events observed • High - dose cohort fully enrolled with results expected 2H 2021 Executive Summary: AVTX - 002 Demonstrates Potential Proof - of - Concept in Initial Low - Dose Cohort *TNF α , tumor necrosis factor alpha; † SES - CD, Simple Endoscopic Score for Crohn’s Disease. 2nd Positive Proof - of - Concept Study With AVTX - 002 Further Validates the LIGHT MOA in Inflammatory Diseases
9 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • Inflammatory bowel disease (IBD) is a broad term indicating chronic inflammation of the gastrointestinal (GI) tract and includes both Crohn’s disease (CD) and ulcerative colitis (UC) 1 – Relapsing and remitting course characterized by intestinal inflammation and epithelial injury, causing lifelong morbidity 2 that significantly impacts quality of life 3,4 • Standard of care relies on treating the inflammatory activity that causes strictures, fistula, and abscesses as well as heightened incidence of colitis - associated neoplasia associated with IBD 5 • An estimated 1.6 - 3.1 million US adults (~1.3%) have a diagnosis of IBD 1,6 – Estimated US cases of CD are ~785K 7 • Approximately $16.7B global market opportunity in 2020 with 4.5% compound annual growth rate 8 Inflammatory Bowel Disease Overview 1. Centers for Disease Control and Prevention. Inflammatory bowel disease. https://www.cdc.gov/ibd/features/IBD - more - chronic - dis eases.html. Accessed July 17, 2021. 2. Atreya R et al. Front Med (Lausanne). 2020;7:517. 3. Knowles SR et al. Inflamm Bowel Dis . 2018;24(4):742 - 751. 4. Byron C et al. J Clin Nurs . 2020;29(3 - 4):305 - 319. 5. GBD 2017 Inflammatory Bowel Disease Collaborators. Lancet . 2020;5:17 - 30. 6. Crohn’s and Colitis Foundation of America. The facts about inflammatory bowel disease. https://www.crohnscolitisfoundatio n.o rg/sites/default/files/2019 - 02/Updated%20IBD%20Factbook.pdf. Accessed July 19, 2021. 7. Shivashankar R et al. Clin Gastroenterol Hepatol. 2017;15(6):857 - 863. 8. EMR Reports. https://www.expertmarketresearch.com/reports/inflammatory - bowel - disease - treatment - market. Ac cessed July 17, 2021. Significant Unmet Need Exists in Crohn’s Disease and Ulcerative Colitis
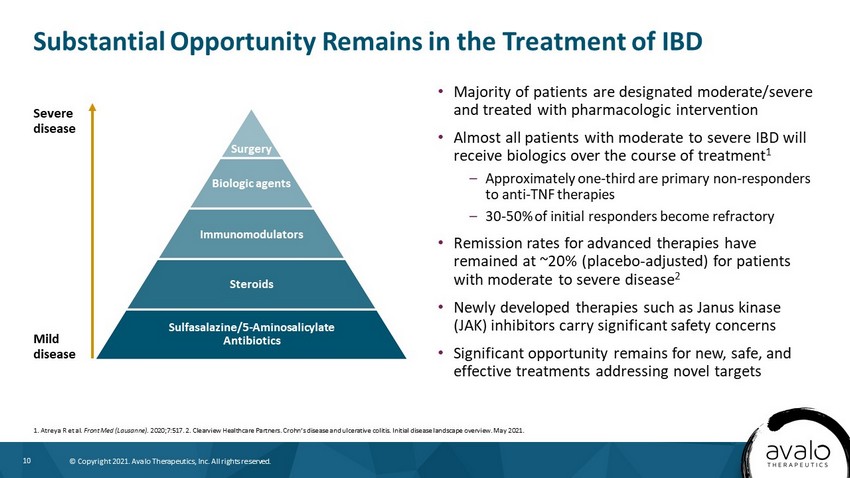
10 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Substantial Opportunity Remains in the Treatment of IBD • Majority of patients are designated moderate/severe and treated with pharmacologic intervention • Almost all patients with moderate to severe IBD will receive biologics over the course of treatment 1 – Approximately one - third are primary non - responders to anti - TNF therapies – 30 - 50% of initial responders become refractory • Remission rates for advanced therapies have remained at ~20% (placebo - adjusted) for patients with moderate to severe disease 2 • Newly developed therapies such as Janus kinase (JAK) inhibitors carry significant safety concerns • Significant opportunity remains for new, safe, and effective treatments addressing novel targets 1. Atreya R et al. Front Med (Lausanne). 2020;7:517. 2. Clearview Healthcare Partners. Crohn’s disease and ulcerative colitis. Initial disease landscape overview. May 20 21. Severe disease Mild disease Surgery Biologic agents Immunomodulators Steroids Sulfasalazine/5 - Aminosalicylate Antibiotics
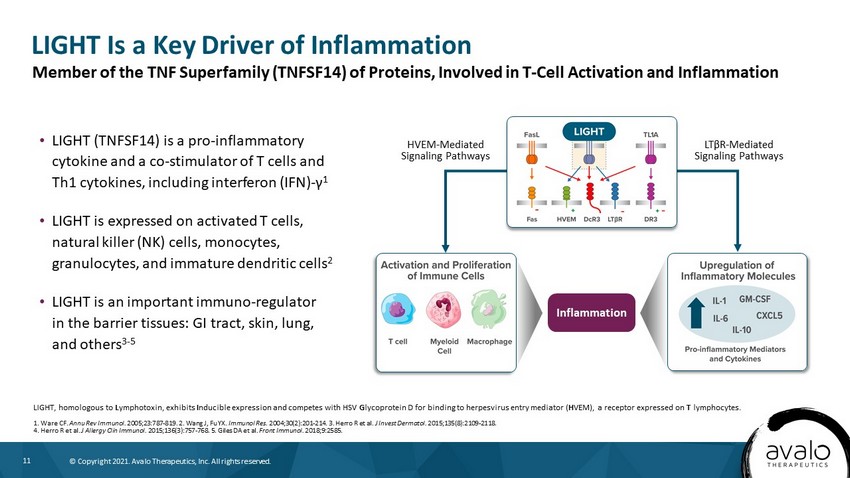
11 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. LIGHT Is a Key Driver of Inflammation LIGHT, homologous to L ymphotoxin, exhibits I nducible expression and competes with HSV G lycoprotein D for binding to herpesvirus entry mediator ( H VEM), a receptor expressed on T lymphocytes. 1. Ware CF. Annu Rev Immunol. 2005;23:787 - 819. 2. Wang J, Fu YX. Immunol Res. 2004;30(2):201 - 214. 3. Herro R et al. J Invest Dermatol. 2015;135(8):2109 - 2118. 4. Herro R et al. J Allergy Clin Immunol. 2015;136(3):757 - 768. 5. Giles DA et al. Front Immunol. 2018;9:2585. • LIGHT (TNFSF14) is a pro - inflammatory cytokine and a co - stimulator of T cells and Th1 cytokines, including interferon (IFN) - γ 1 • LIGHT is expressed on activated T cells, natural killer (NK) cells, monocytes, granulocytes, and immature dendritic cells 2 • LIGHT is an important immuno - regulator in the barrier tissues: GI tract, skin, lung, and others 3 - 5 Member of the TNF Superfamily (TNFSF14) of Proteins, Involved in T - Cell Activation and Inflammation Inflammation HVEM - Mediated Signaling Pathways LT β R - Mediated Signaling Pathways
12 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Multiple Lines of Evidence Support Importance of LIGHT in IBD 1. Data on file, Avalo Therapeutics, Inc. 2. Moraes L et al. Inflamm Bowel Dis. 2020;26(6):874 - 884. 3. Cohavy O et al. J Immunol. 2005;174(2):646 - 653. 4. Wang J et al. J Immunol. 2005;174(12):8173 - 8182. 5. Shaikh RB et al. J Immunol. 2001;167(11):6330 - 6337. 6. Jungbeck M et al. Immunology . 2009;128(3):451 - 458. 7. Schaer C et al. PLoS One. 2011;6(4):e18495. • Patient data – Elevated levels of LIGHT in patients with CD 1 and UC 2 – High LIGHT mRNA levels detected in human inflamed intestinal tissue compared with normal tissue 1,3 – LIGHT gene upregulation is observed in CD and UC 4 • Animal models of IBD – LIGHT overexpression increases intestinal inflammation in rodents 5 – Anti - LIGHT monoclonal antibody (mAb) treatment ameliorates inflammation in the dextrate sulfate sodium (DSS) – induced colitis model 6 – Knockout of the LIGHT (or its ligand, HVEM) gene results in reduced intestinal inflammation (in some models) 7
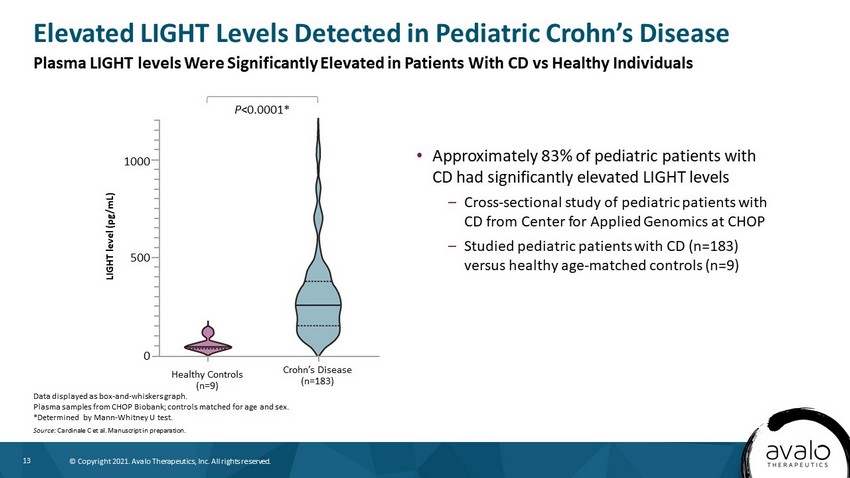
13 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • Approximately 83% of pediatric patients with CD had significantly elevated LIGHT levels – Cross - sectional study of pediatric patients with CD from Center for Applied Genomics at CHOP – Studied pediatric patients with CD (n=183) versus healthy age - matched controls (n=9) Elevated LIGHT Levels Detected in Pediatric Crohn’s Disease Data displayed as box - and - whiskers graph. Plasma samples from CHOP Biobank; controls matched for age and sex. *Determined by Mann - Whitney U test. Source : Cardinale C et al. Manuscript in preparation. LIGHT level (pg/mL) Healthy Controls (n=9) Crohn’s Disease (n=183) 1000 500 0 P <0.0001* Plasma LIGHT levels Were Significantly Elevated in Patients With CD vs Healthy Individuals
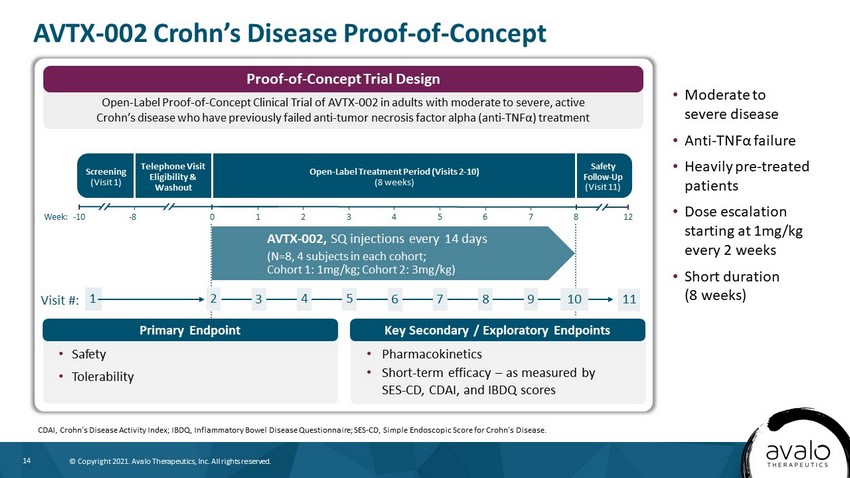
14 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • Moderate to severe disease • Anti - TNF α failure • Heavily pre - treated patients • Dose escalation starting at 1mg/kg every 2 weeks • Short duration (8 weeks) AVTX - 002 Crohn’s Disease Proof - of - Concept CDAI, Crohn’s Disease Activity Index; IBDQ, Inflammatory Bowel Disease Questionnaire; SES - CD, Simple Endoscopic Score for Crohn’ s Disease. Screening (Visit 1) Open - Label Treatment Period (Visits 2 - 10) (8 weeks) Safety Follow - Up (Visit 11) AVTX - 002, SQ injections every 14 days (N=8, 4 subjects in each cohort; Cohort 1: 1mg/kg; Cohort 2: 3mg/kg) Telephone Visit Eligibility & Washout Week: - 10 0 1 2 3 4 5 6 - 8 12 7 8 Visit #: 1 2 3 4 5 6 7 10 8 9 11 • Safety • Tolerability • Pharmacokinetics • Short - term efficacy – as measured by SES - CD, CDAI, and IBDQ scores Open - Label Proof - of - Concept Clinical Trial of AVTX - 002 in adults with moderate to severe, active Crohn’s disease who have previously failed anti - tumor necrosis factor alpha (anti - TNF α) treatment Proof - of - Concept Trial Design Primary Endpoint Key Secondary / Exploratory Endpoints
15 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Clinical Trials in CD Consistently Suggest More Severe Patients Are Less Likely to Achieve Spontaneous Remission PBO, placebo; KOL, key opinion leader. Source: Su . Gastroenterology. 2004; Su . Gastroenterology. 2007; Physician Interviews; ClearView Analysis. Key Takeaways from KOL interviews - Placebo Response and Remission in CD • Gastroenterology KOLs (N=6) noted that they expect low PBO rates in heavily pre - treated patient populations (as low as single - digits in 4L+ patients) • Patients with more severe disease are less likely to have spontaneous remission • Studies using endoscopic healing as a primary endpoint have lower PBO response and remission rates vs. those that use symptomatic endpoints (e.g., CDAI) • Studies with shorter treatment duration have lower PBO response rates vs. those with longer treatment duration
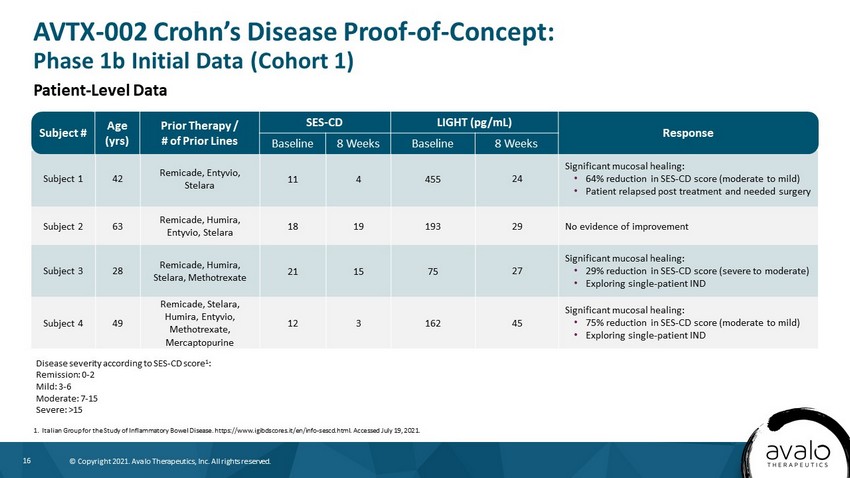
16 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Subject 1 42 Remicade, Entyvio, Stelara 11 4 455 24 Significant mucosal healing: • 64% reduction in SES - CD score (moderate to mild) • Patient relapsed post treatment and needed surgery Subject 2 63 Remicade, Humira, Entyvio, Stelara 18 19 193 29 No evidence of improvement Subject 3 28 Remicade, Humira, Stelara, Methotrexate 21 15 75 27 Significant mucosal healing: • 29% reduction in SES - CD score (severe to moderate) • Exploring single - patient IND Subject 4 49 Remicade, Stelara, Humira, Entyvio, Methotrexate, Mercaptopurine 12 3 162 45 Significant mucosal healing: • 75% reduction in SES - CD score (moderate to mild) • Exploring single - patient IND Subject # Age (yrs) Prior Therapy / # of Prior Lines SES - CD LIGHT (pg/mL) Response Baseline 8 Weeks Baseline 8 Weeks AVTX - 002 Crohn’s Disease Proof - of - Concept: Phase 1b Initial Data (Cohort 1) 1. Italian Group for the Study of Inflammatory Bowel Disease. https://www.igibdscores.it/en/info - sescd.html. Accessed July 19, 2021. Disease severity according to SES - CD score 1 : Remission: 0 - 2 Mild: 3 - 6 Moderate: 7 - 15 Severe: >15 Patient - Level Data
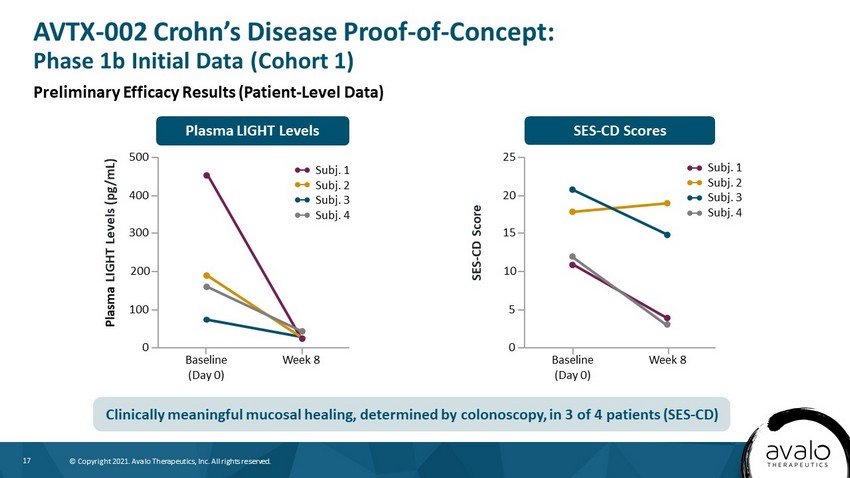
17 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. SES - CD Scores Plasma LIGHT Levels SES - CD Score 25 Baseline (Day 0) Week 8 20 10 5 0 15 Plasma LIGHT Levels (pg/mL) 500 Baseline (Day 0) Week 8 400 200 100 0 300 Subj. 1 Subj. 2 Subj. 3 Subj. 4 Subj. 1 Subj. 2 Subj. 3 Subj. 4 AVTX - 002 Crohn’s Disease Proof - of - Concept: Phase 1b Initial Data (Cohort 1) Preliminary Efficacy Results (Patient - Level Data) Clinically meaningful mucosal healing, determined by colonoscopy, in 3 of 4 patients (SES - CD)
18 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • No serious adverse events attributable to study drug – Consistent with 83 - patient COVID - 19 ARDS clinical trial 1 • Adverse events were mild to moderate in nature – Most common: GI symptoms consistent with CD • No evidence of increased infections or adverse events related to immunosuppression • Recommended by independent safety review committee to continue to next cohort (3mg/kg) without changes to protocol (currently fully enrolled) AVTX - 002 Crohn’s Disease Proof - of - Concept: Phase 1b Initial Data (Cohort 1) 1. Perlin DS et al. AVTX - 002, a human anti - LIGHT mAb reduces respiratory failure and death in hospitalized COVID - 19 ARDS patient s (https://medrxiv.org/cgi/content/short/2021.04.03.21254748v1). Independent Preliminary Safety Data Results – AVTX - 002, SQ Injection (1mg/kg)
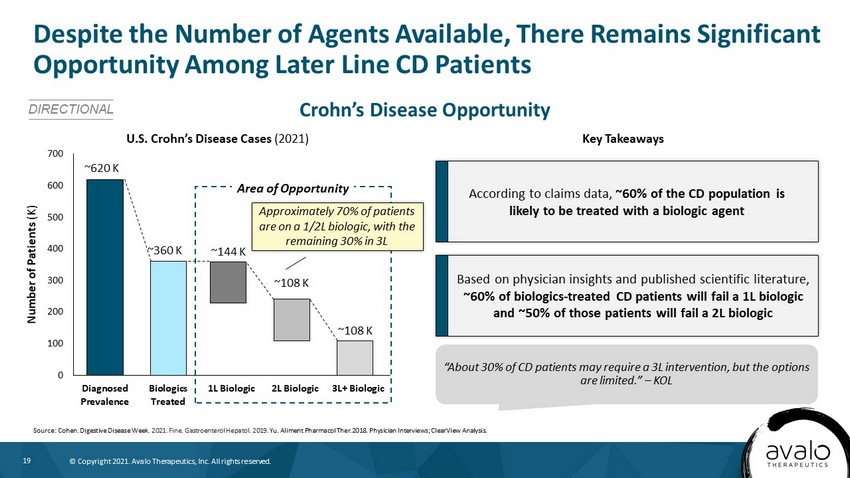
19 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Crohn’s Disease Opportunity Despite the Number of Agents Available, There Remains Significant Opportunity Among Later Line CD Patients DIRECTIONAL U.S. Crohn’s Disease Cases (2021) Key Takeaways According to claims data, ~60% of the CD population is likely to be treated with a biologic agent Based on physician insights and published scientific literature, ~60% of biologics - treated CD patients will fail a 1L biologic and ~50% of those patients will fail a 2L biologic “About 30% of CD patients may require a 3L intervention, but the options are limited.” – KOL Source: Cohen. Digestive Disease Week . 2021. Fine. Gastroenterol Hepatol. 2019. Yu. Aliment Pharmacol Ther.2018. Physician Interviews; ClearView Analysis. 0 100 200 300 400 500 600 700 Diagnosed Prevalence Biologics Treated 1L Biologic 2L Biologic 3L+ Biologic Number of Patients (K) ~360 K ~620 K ~144 K ~108 K ~108 K Area of Opportunity Approximately 70% of patients are on a 1/2L biologic, with the remaining 30% in 3L

20 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. 20 AVTX - 002: COVID - 19 ARDS
21 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • AVTX - 002 significantly reduced respiratory failure and mortality in a Phase 2 clinical trial in patients hospitalized with COVID - 19 acute respiratory distress syndrome (ARDS) – This analysis updates the preliminary top - line data reported on January 5, 2021, and is inclusive of 60 - day safety data – Hospitalized COVID - 19 patients treated with a single dose of AVTX - 002 demonstrated statistically significant improvement in the primary endpoint (proportion of patients alive and free of respiratory failure over the 28 - day study period) compared with placebo (n=62, P =0.044) – Efficacy was highest in a prespecified subpopulation of patients aged ≥60 years (n=34, P =0.042), the population most vulnerable to severe complications and death with COVID - 19 infection – At both the 28 - day and 60 - day final timepoints, an approximate 50% trend in mortality reduction (22.5% vs 10.8%) was observed – AVTX - 002 showed statistically significant efficacy on top of corticosteroids and standard of care in COVID - 19 ARDS (~88% of patients in the trial received corticosteroids and ~58% received remdesivir) • AVTX - 002 was well tolerated, with no appreciable differences in immunosuppression or other serious adverse events between AVTX - 002 and placebo • AVTX - 002 dramatically and rapidly reduced serum free - LIGHT levels – ~85% reduction in free LIGHT achieved in 1 day • AVTX - 002 recently granted Fast Track designation for the treatment of hospitalized patients with COVID - 19 • Additionally, the Company is exploring the applicability of AVTX - 002 in non - COVID - 19 ARDS Executive Summary: Final Data Analysis Phase 2 Clinical Trial Met Primary Endpoint in Patients Hospitalized With COVID - 19 ARDS Source: Data on file, Avalo Therapeutics, Inc.
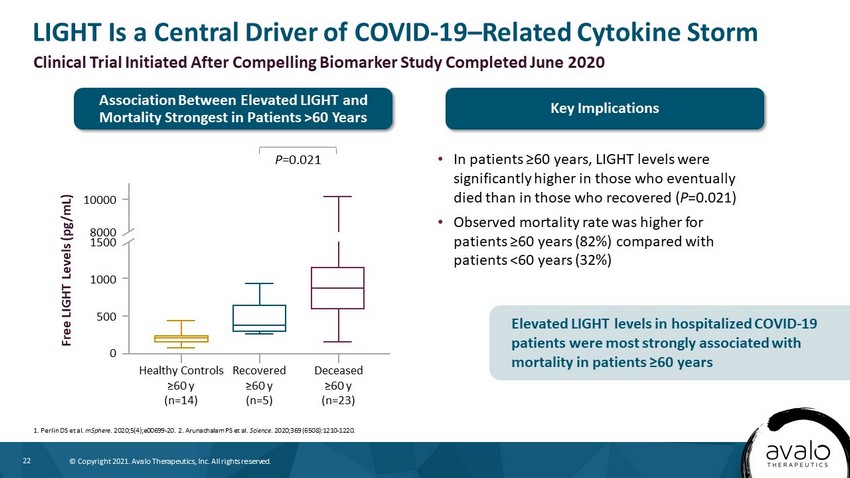
22 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. LIGHT Is a Central Driver of COVID - 19 – Related Cytokine Storm • In patients ≥60 years, LIGHT levels were significantly higher in those who eventually died than in those who recovered ( P =0.021) • Observed mortality rate was higher for patients ≥60 years (82%) compared with patients <60 years (32%) 1. Perlin DS et al. mSphere . 2020;5(4);e00699 - 20. 2. Arunachalam PS et al. Science . 2020;369 (6508):1210 - 1220. Clinical Trial Initiated After Compelling Biomarker Study Completed June 2020 Free LIGHT Levels (pg/mL) 10000 8000 1500 1000 500 0 Healthy Controls ≥60 y (n=14) Recovered ≥60 y (n=5) Deceased ≥60 y (n=23) P =0.021 Association Between Elevated LIGHT and Mortality Strongest in Patients >60 Years Key Implications Elevated LIGHT levels in hospitalized COVID - 19 patients were most strongly associated with mortality in patients ≥60 years
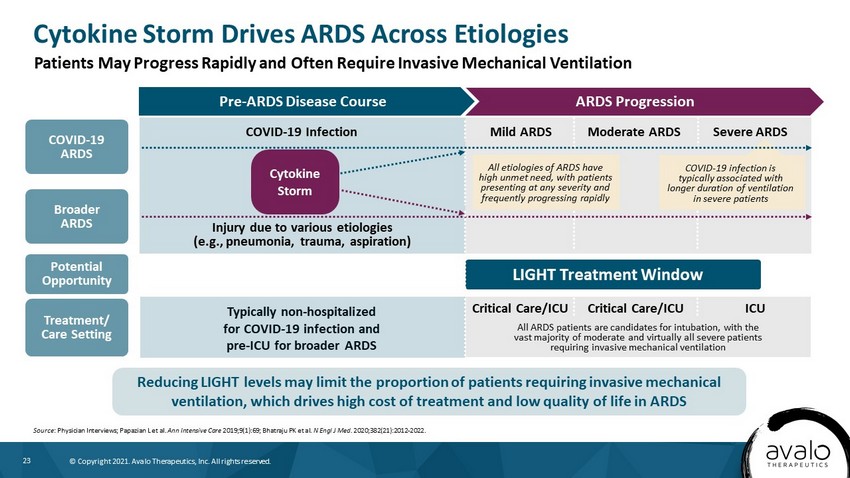
23 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. ARDS Progression Pre - ARDS Disease Course Cytokine Storm Drives ARDS Across Etiologies Source : Physician Interviews; Papazian L et al. Ann Intensive Care 2019;9(1):69; Bhatraju PK et al. N Engl J Med. 2020;382(21):2012 - 2022. Patients May Progress Rapidly and Often Require Invasive Mechanical Ventilation Reducing LIGHT levels may limit the proportion of patients requiring invasive mechanical ventilation, which drives high cost of treatment and low quality of life in ARDS All ARDS patients are candidates for intubation, with the vast majority of moderate and virtually all severe patients requiring invasive mechanical ventilation Critical Care/ICU ICU Critical Care/ICU COVID - 19 Infection Injury due to various etiologies (e.g., pneumonia, trauma, aspiration) Typically non - hospitalized for COVID - 19 infection and pre - ICU for broader ARDS COVID - 19 ARDS Mild ARDS Moderate ARDS Severe ARDS Cytokine Storm Broader ARDS Potential Opportunity Treatment/ Care Setting LIGHT Treatment Window All etiologies of ARDS have high unmet need, with patients presenting at any severity and frequently progressing rapidly COVID - 19 infection is typically associated with longer duration of ventilation in severe patients
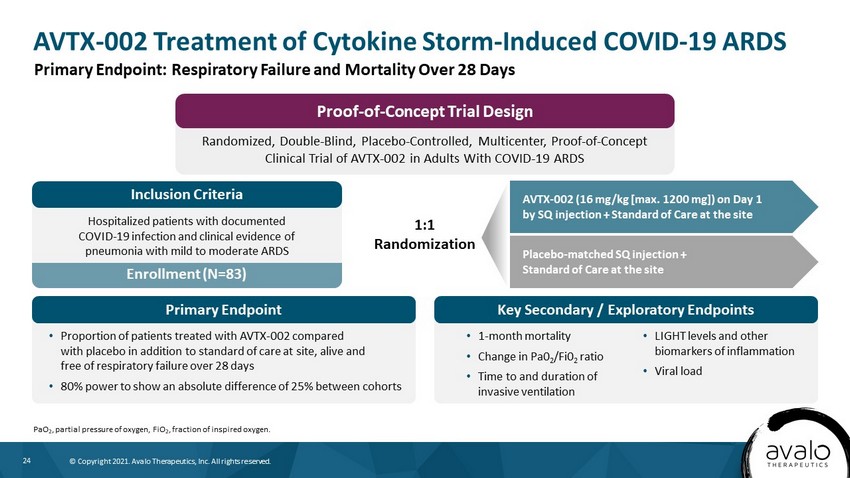
24 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. AVTX - 002 Treatment of Cytokine Storm - Induced COVID - 19 ARDS PaO 2 , partial pressure of oxygen, FiO 2 , fraction of inspired oxygen. Primary Endpoint: Respiratory Failure and Mortality Over 28 Days Randomized, Double - Blind, Placebo - Controlled, Multicenter, Proof - of - Concept Clinical Trial of AVTX - 002 in Adults With COVID - 19 ARDS Proof - of - Concept Trial Design Enrollment (N=83) Hospitalized patients with documented COVID - 19 infection and clinical evidence of pneumonia with mild to moderate ARDS Inclusion Criteria • Proportion of patients treated with AVTX - 002 compared with placebo in addition to standard of care at site, alive and free of respiratory failure over 28 days • 80% power to show an absolute difference of 25% between cohorts Primary Endpoint Key Secondary / Exploratory Endpoints • 1 - month mortality • Change in Pa0 2 /Fi0 2 ratio • Time to and duration of invasive ventilation • LIGHT levels and other biomarkers of inflammation • Viral load AVTX - 002 (16 mg/kg [max. 1200 mg]) on Day 1 by SQ injection + Standard of Care at the site 1:1 Randomization Placebo - matched SQ injection + Standard of Care at the site
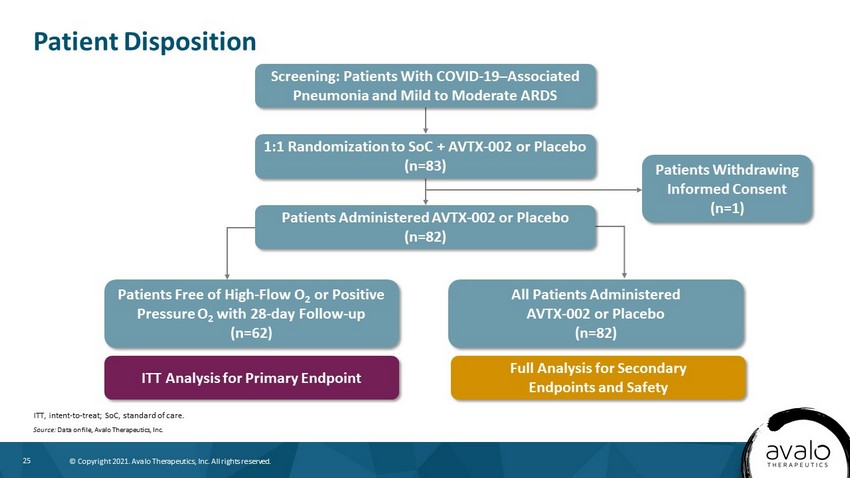
25 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Patient Disposition ITT, intent - to - treat; SoC, standard of care. Source: Data on file, Avalo Therapeutics, Inc. Screening: Patients With COVID - 19 – Associated Pneumonia and Mild to Moderate ARDS 1:1 Randomization to SoC + AVTX - 002 or Placebo (n=83) Patients Administered AVTX - 002 or Placebo (n=82) Patients Withdrawing Informed Consent (n=1) Patients Free of High - Flow O 2 or Positive Pressure O 2 with 28 - day Follow - up (n=62) All Patients Administered AVTX - 002 or Placebo (n=82) ITT Analysis for Primary Endpoint Full Analysis for Secondary Endpoints and Safety
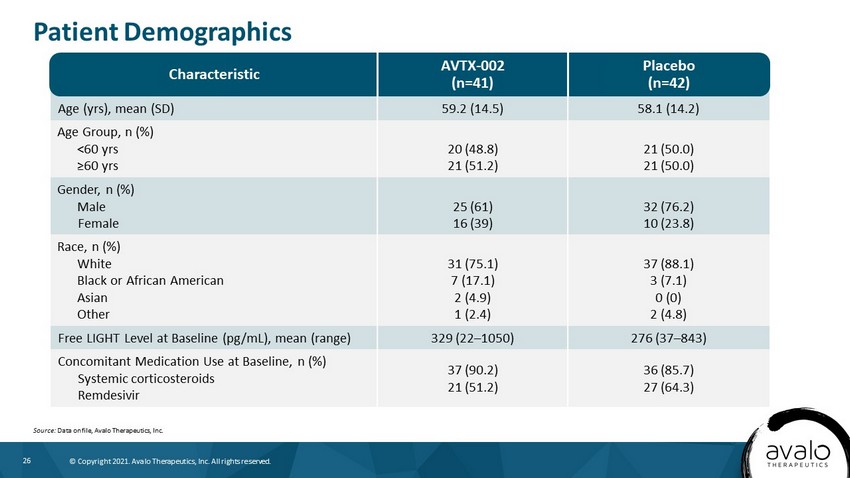
26 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Age (yrs), mean (SD) 59.2 (14.5) 58.1 (14.2) Age Group, n (%) <60 yrs ≥60 yrs 20 (48.8) 21 (51.2) 21 (50.0) 21 (50.0) Gender, n (%) Male Female 25 (61) 16 (39) 32 (76.2) 10 (23.8) Race, n (%) White Black or African American Asian Other 31 (75.1) 7 (17.1) 2 (4.9) 1 (2.4) 37 (88.1) 3 (7.1) 0 (0) 2 (4.8) Free LIGHT Level at Baseline (pg/mL), mean (range) 329 (22 – 1050) 276 (37 – 843) Concomitant Medication Use at Baseline, n (%) Systemic corticosteroids Remdesivir 37 (90.2) 21 (51.2) 36 (85.7) 27 (64.3) Patient Demographics Source: Data on file, Avalo Therapeutics, Inc. AVTX - 002 (n=41) Characteristic Placebo (n=42)
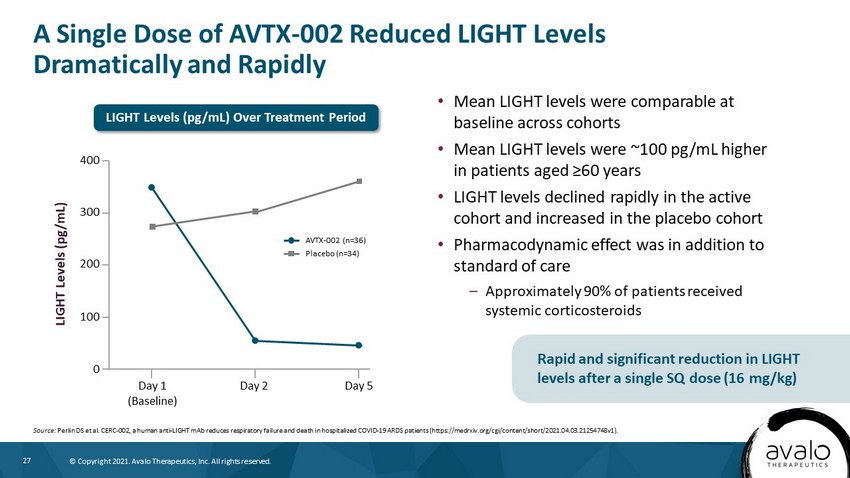
27 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. A Single Dose of AVTX - 002 Reduced LIGHT Levels Dramatically and Rapidly • Mean LIGHT levels were comparable at baseline across cohorts • Mean LIGHT levels were ~100 pg/mL higher in patients aged ≥60 years • LIGHT levels declined rapidly in the active cohort and increased in the placebo cohort • Pharmacodynamic effect was in addition to standard of care – Approximately 90% of patients received systemic corticosteroids Source: Perlin DS et al. CERC - 002, a human anti - LIGHT mAb reduces respiratory failure and death in hospitalized COVID - 19 ARDS patients ( https://medrxiv.org/cgi/content/short/2021.04.03.21254748v1). Rapid and significant reduction in LIGHT levels after a single SQ dose (16 mg/kg) AVTX - 002 (n=36) Placebo (n=34) LIGHT Levels (pg/mL) 400 300 200 100 0 Day 5 Day 1 (Baseline) Day 2 LIGHT Levels (pg/mL) Over Treatment Period
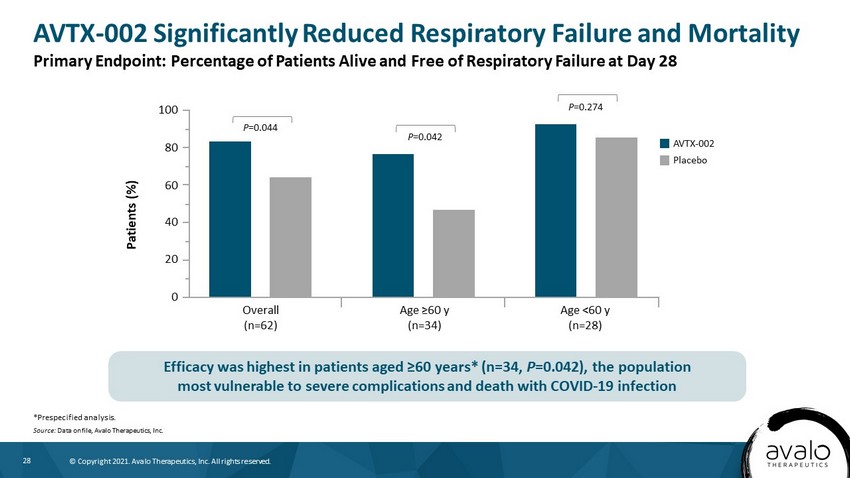
28 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. AVTX - 002 Significantly Reduced Respiratory Failure and Mortality *Prespecified analysis. Source: Data on file, Avalo Therapeutics, Inc. Primary Endpoint: Percentage of Patients Alive and Free of Respiratory Failure at Day 28 Efficacy was highest in patients aged ≥60 years* (n=34, P =0.042), the population most vulnerable to severe complications and death with COVID - 19 infection Patients (%) 100 60 40 20 0 AVTX - 002 Overall (n=62) Placebo Age ≥60 y (n=34) Age <60 y (n=28) 80 P =0.044 P =0.042 P =0.274
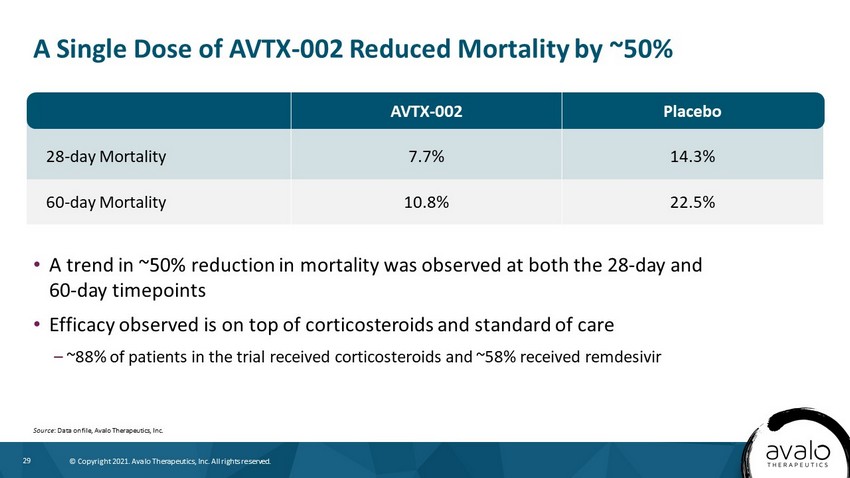
29 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. 28 - day Mortality 7.7% 14.3% 60 - day Mortality 10.8% 22.5% A Single Dose of AVTX - 002 Reduced Mortality by ~50% Source : Data on file, Avalo Therapeutics, Inc. • A trend in ~50% reduction in mortality was observed at both the 28 - day and 60 - day timepoints • Efficacy observed is on top of corticosteroids and standard of care – ~88% of patients in the trial received corticosteroids and ~58% received remdesivir AVTX - 002 Placebo
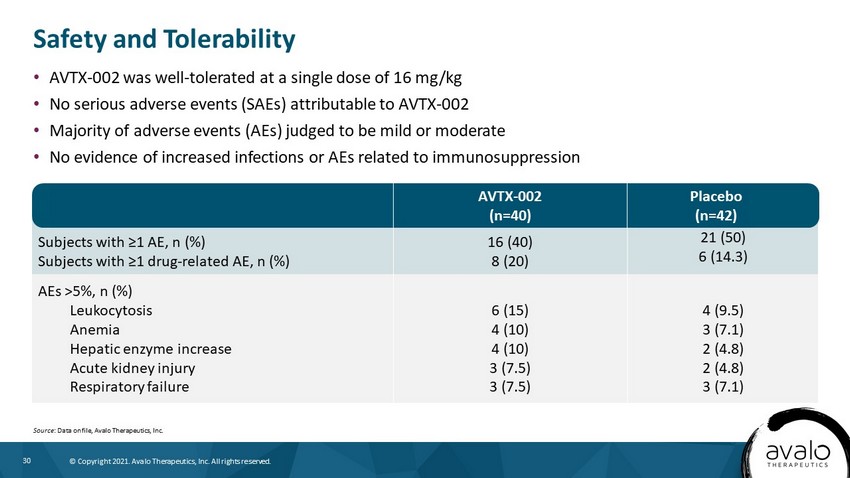
30 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Subjects with ≥1 AE, n (%) Subjects with ≥1 drug - related AE, n (%) 16 (40) 8 (20) 21 (50) 6 (14.3) AEs >5%, n (%) Leukocytosis Anemia Hepatic enzyme increase Acute kidney injury Respiratory failure 6 (15) 4 (10) 4 (10) 3 (7.5) 3 (7.5) 4 (9.5) 3 (7.1) 2 (4.8) 2 (4.8) 3 (7.1) • AVTX - 002 was well - tolerated at a single dose of 16 mg/kg • No serious adverse events (SAEs) attributable to AVTX - 002 • Majority of adverse events (AEs) judged to be mild or moderate • No evidence of increased infections or AEs related to immunosuppression Safety and Tolerability Source : Data on file, Avalo Therapeutics, Inc. AVTX - 002 (n=40) Placebo (n=42)
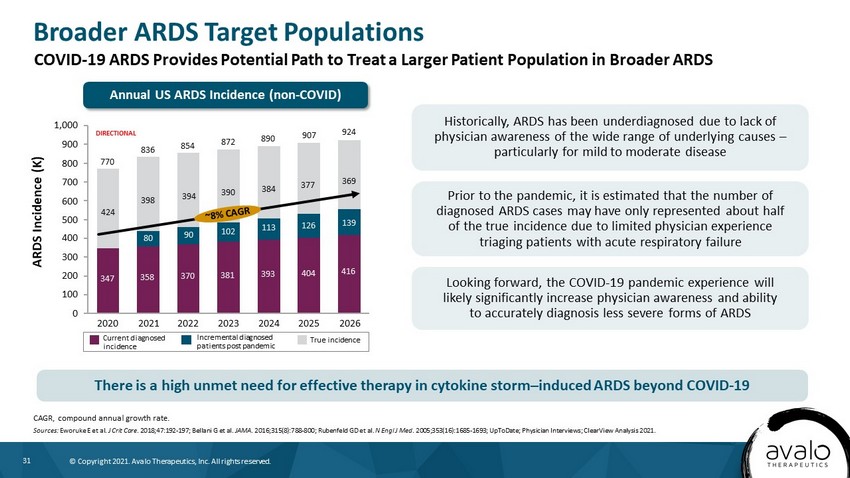
31 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Broader ARDS Target Populations CAGR, compound annual growth rate. Sources: Eworuke E et al. J Crit Care . 2018;47:192 - 197; Bellani G et al. JAMA . 2016;315(8):788 - 800; Rubenfeld GD et al. N Engl J Med . 2005;353(16):1685 - 1693; UpToDate; Physician Interviews; ClearView Analysis 2021. ARDS Incidence (K) Historically, ARDS has been underdiagnosed due to lack of physician awareness of the wide range of underlying causes – particularly for mild to moderate disease Prior to the pandemic, it is estimated that the number of diagnosed ARDS cases may have only represented about half of the true incidence due to limited physician experience triaging patients with acute respiratory failure Looking forward, the COVID - 19 pandemic experience will likely significantly increase physician awareness and ability to accurately diagnosis less severe forms of ARDS 400 200 800 300 500 0 100 600 1,000 700 900 90 2023 381 854 424 924 2026 347 398 2020 80 358 2021 394 370 770 393 2022 390 102 384 113 2024 416 139 126 2025 369 377 872 404 890 907 836 Current diagnosed incidence Incremental diagnosed patients post pandemic True incidence DIRECTIONAL COVID - 19 ARDS Provides Potential Path to Treat a Larger Patient Population in Broader ARDS There is a high unmet need for effective therapy in cytokine storm – induced ARDS beyond COVID - 19 Annual US ARDS Incidence (non - COVID)
32 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • Crohn’s Disease – Further evaluation of cohort data, including tissue LIGHT levels – Cohort 2 (3 mg/kg dose) fully enrolled; complete data anticipated in 2H 2021 • Ulcerative Colitis – Expand clinical study to patients with moderate to severe UC refractory to anti - TNF α * • ARDS Program – Continuing dialogue with FDA to determine registration trial design and timing, including potential expansion to broader ARDS patient population • Exploring additional indications for disease in which LIGHT is a driver of inflammation AVTX - 002 Clinical Program *TNF α , tumor necrosis factor alpha. Next Steps

33 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. 33 Phase 1b anti - IL - 18 monoclonal antibody for Multiple Myeloma and Still’s Disease (AOSD and sJIA) AVTX - 007
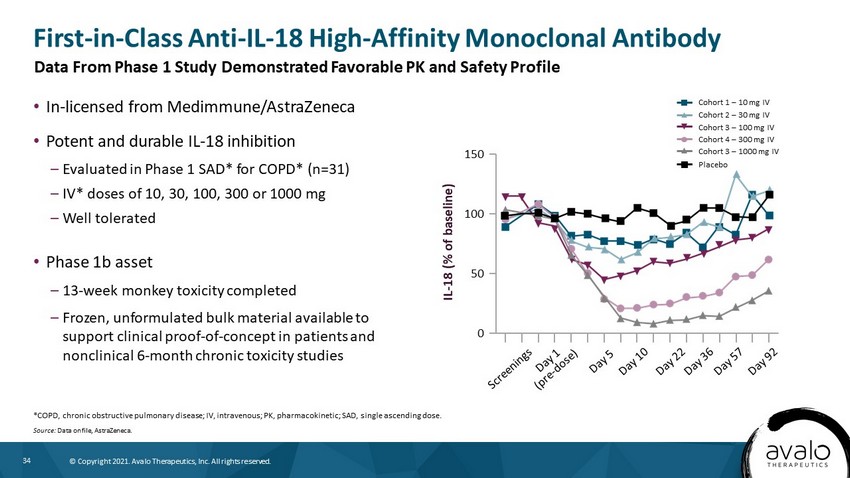
34 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. IL - 18 (% of baseline) 150 100 50 0 • In - licensed from Medimmune/AstraZeneca • Potent and durable IL - 18 inhibition – Evaluated in Phase 1 SAD* for COPD* (n=31) – IV* doses of 10, 30, 100, 300 or 1000 mg – Well tolerated • Phase 1b asset – 13 - week monkey toxicity completed – Frozen, unformulated bulk material available to support clinical proof - of - concept in patients and nonclinical 6 - month chronic toxicity studies First - in - Class Anti - IL - 18 High - Affinity Monoclonal Antibody *COPD, chronic obstructive pulmonary disease; IV, intravenous; PK, pharmacokinetic; SAD, single ascending dose. Source: Data on file, AstraZeneca. Data From Phase 1 Study Demonstrated Favorable PK and Safety Profile Cohort 1 – 10 mg IV Cohort 2 – 30 mg IV Cohort 3 – 100 mg IV Cohort 4 – 300 mg IV Cohort 3 – 1000 mg IV Placebo
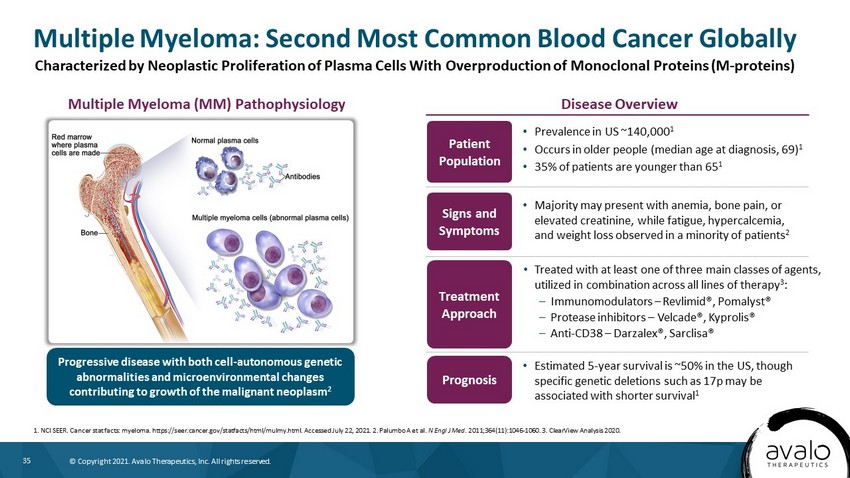
35 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Multiple Myeloma: Second Most Common Blood Cancer Globally 1. NCI SEER. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 22, 2021. 2. Palumb o A et al. N Engl J Med . 2011;364(11):1046 - 1060. 3. ClearView Analysis 2020. Treatment Approach • Majority may present with anemia, bone pain, or elevated creatinine, while fatigue, hypercalcemia, and weight loss observed in a minority of patients 2 Prognosis • Estimated 5 - year survival is ~50% in the US, though specific genetic deletions such as 17p may be associated with shorter survival 1 Patient Population • Prevalence in US ~140,000 1 • Occurs in older people (median age at diagnosis, 69) 1 • 35% of patients are younger than 65 1 Progressive disease with both cell - autonomous genetic abnormalities and microenvironmental changes contributing to growth of the malignant neoplasm 2 • Treated with at least one of three main classes of agents, utilized in combination across all lines of therapy 3 : – Immunomodulators – Revlimid®, Pomalyst® – Protease inhibitors – Velcade®, Kyprolis® – Anti - CD38 – Darzalex®, Sarclisa® Disease Overview Multiple Myeloma (MM) Pathophysiology Characterized by Neoplastic Proliferation of Plasma Cells With Overproduction of Monoclonal Proteins (M - proteins) Signs and Symptoms
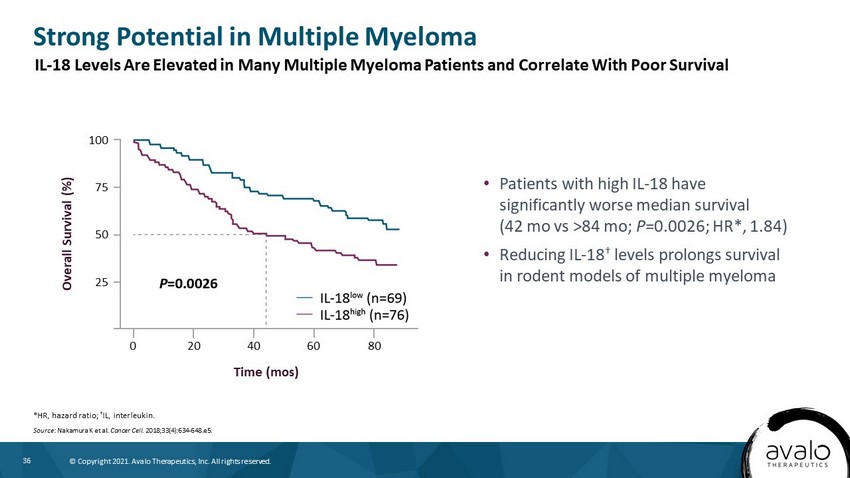
36 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Strong Potential in Multiple Myeloma *HR, hazard ratio; † IL, interleukin . Source : Nakamura K et al. Cancer Cell. 2018;33(4):634 - 648.e5. • Patients with high IL - 18 have significantly worse median survival (42 mo vs >84 mo; P =0.0026; HR*, 1.84) • Reducing IL - 18 † levels prolongs survival in rodent models of multiple myeloma Overall Survival (%) 100 75 50 0 25 Time (mos) 20 40 60 80 IL - 18 Levels Are Elevated in Many Multiple Myeloma Patients and Correlate With Poor Survival
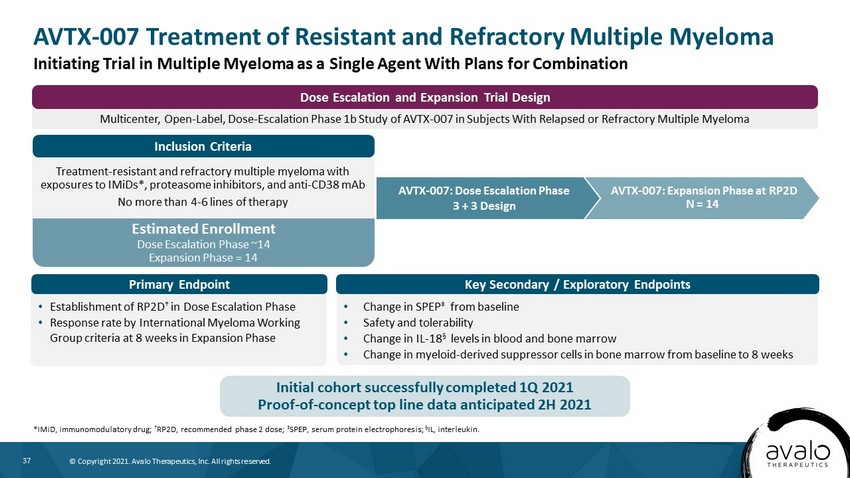
37 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Estimated Enrollment Dose Escalation Phase ~14 Expansion Phase = 14 Multicenter, Open - Label, Dose - Escalation Phase 1b Study of AVTX - 007 in Subjects With Relapsed or Refractory Multiple Myeloma Dose Escalation and Expansion Trial Design AVTX - 007 Treatment of Resistant and Refractory Multiple Myeloma *IMiD, immunomodulatory drug; † RP2D, recommended phase 2 dose; ‡ SPEP, serum protein electrophoresis; Α IL, interleukin. AVTX - 007: Expansion Phase at RP2D N = 14 Treatment - resistant and refractory multiple myeloma with exposures to IMiDs*, proteasome inhibitors, and anti - CD38 mAb No more than 4 - 6 lines of therapy • Establishment of RP2D † in Dose Escalation Phase • Response rate by International Myeloma Working Group criteria at 8 weeks in Expansion Phase • Change in SPEP ‡ from baseline • Safety and tolerability • Change in IL - 18 § levels in blood and bone marrow • Change in myeloid - derived suppressor cells in bone marrow from baseline to 8 weeks AVTX - 007: Dose Escalation Phase 3 + 3 Design Initiating Trial in Multiple Myeloma as a Single Agent With Plans for Combination Initial cohort successfully completed 1Q 2021 Proof - of - concept top line data anticipated 2H 2021 Primary Endpoint Key Secondary / Exploratory Endpoints Inclusion Criteria
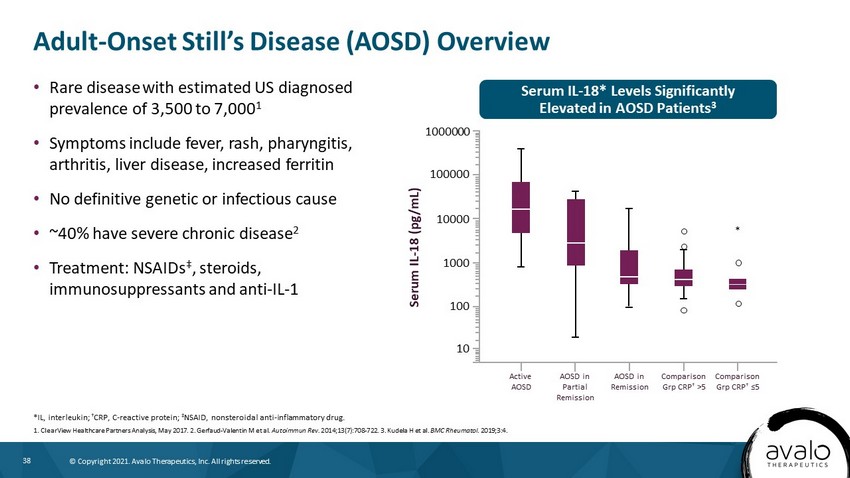
38 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Serum IL - 18* Levels Significantly Elevated in AOSD Patients 3 Adult - Onset Still’s Disease (AOSD) Overview • Rare disease with estimated US diagnosed prevalence of 3,500 to 7,000 1 • Symptoms include fever, rash, pharyngitis, arthritis, liver disease, increased ferritin • No definitive genetic or infectious cause • ~40% have severe chronic disease 2 • Treatment: NSAIDs ‡ , steroids, immunosuppressants and anti - IL - 1 *IL, interleukin; † CRP, C - reactive protein; ‡ NSAID, nonsteroidal anti - inflammatory drug. 1. ClearView Healthcare Partners Analysis, May 2017. 2. Gerfaud - Valentin M et al. Autoimmun Rev. 2014;13(7):708 - 722. 3. Kudela H et al. BMC Rheumatol . 2019;3:4. Active AOSD AOSD in Partial Remission AOSD in Remission Comparison Grp CRP † >5 Comparison Grp CRP † ≤5 Serum IL - 18 (pg/mL) 1000000 100000 10000 1000 100 10 *
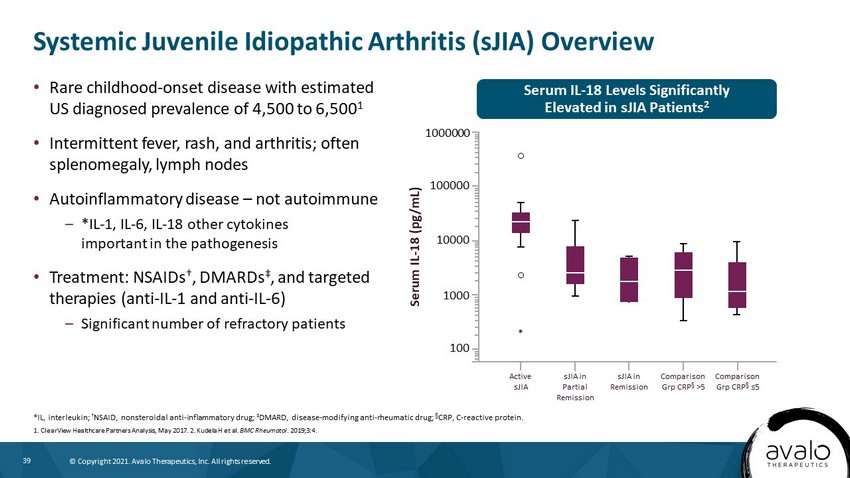
39 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Systemic Juvenile Idiopathic Arthritis (sJIA) Overview *IL, interleukin; † NSAID, nonsteroidal anti - inflammatory drug; ‡ DMARD, disease - modifying anti - rheumatic drug; † CRP, C - reactive protein. 1. ClearView Healthcare Partners Analysis, May 2017. 2. Kudela H et al. BMC Rheumatol . 2019;3:4. • Rare childhood - onset disease with estimated US diagnosed prevalence of 4,500 to 6,500 1 • Intermittent fever, rash, and arthritis; often splenomegaly, lymph nodes • Autoinflammatory disease – not autoimmune – *IL - 1, IL - 6, IL - 18 other cytokines important in the pathogenesis • Treatment: NSAIDs † , DMARDs ‡ , and targeted therapies (anti - IL - 1 and anti - IL - 6) – Significant number of refractory patients Serum IL - 18 Levels Significantly Elevated in sJIA Patients 2 Active sJIA sJIA in Partial Remission sJIA in Remission Comparison Grp CRP † >5 Comparison Grp CRP † ≤5 Serum IL - 18 (pg/mL) 1000000 100000 10000 1000 100 *
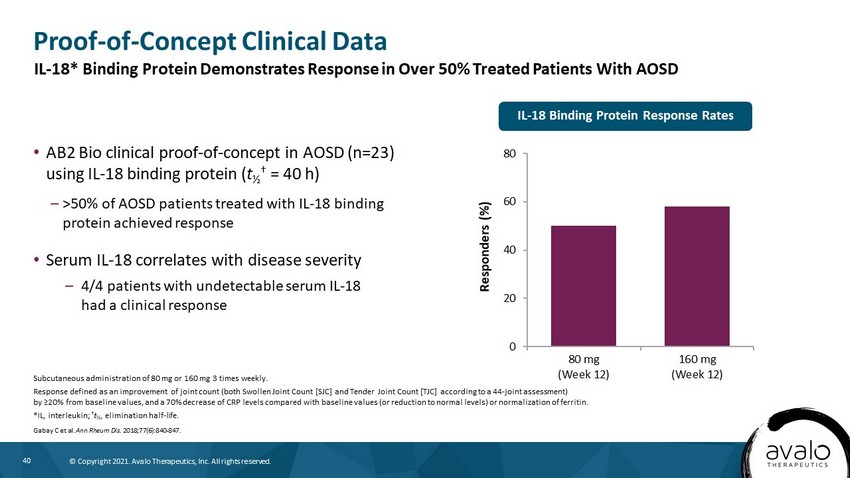
40 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Proof - of - Concept Clinical Data • AB2 Bio clinical proof - of - concept in AOSD (n=23) using IL - 18 binding protein ( t ½ † = 40 h) – >50% of AOSD patients treated with IL - 18 binding protein achieved response • Serum IL - 18 correlates with disease severity – 4/4 patients with undetectable serum IL - 18 had a clinical response Subcutaneous administration of 80 mg or 160 mg 3 times weekly. Response defined as an improvement of joint count (both Swollen Joint Count [SJC] and Tender Joint Count [TJC] according to a 44 - joint assessment) by ≥20% from baseline values, and a 70% decrease of CRP levels compared with baseline values (or reduction to normal levels) or normalization of ferritin. *IL, interleukin; † t ½ , elimination half - life. Gabay C et al. Ann Rheum Dis. 2018;77(6):840 - 847. 0 20 40 60 80 80 mg (Week 12) 160 mg (Week 12) Responders (%) IL - 18 Binding Protein Response Rates IL - 18* Binding Protein Demonstrates Response in Over 50% Treated Patients With AOSD
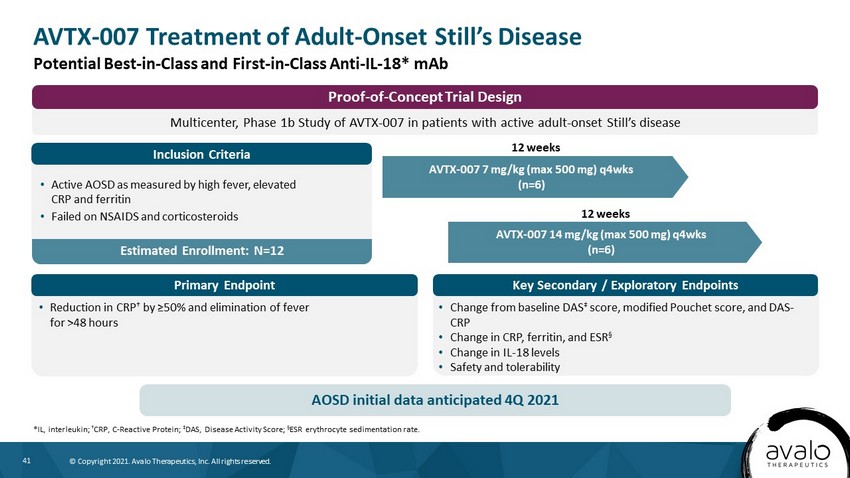
41 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Key Secondary / Exploratory Endpoints Primary Endpoint Estimated Enrollment: N=12 AVTX - 007 7 mg/kg (max 500 mg) q4wks (n=6) *IL, interleukin; † CRP, C - Reactive Protein; ‡ DAS, Disease Activity Score; Α ESR erythrocyte sedimentation rate. 12 weeks • Reduction in CRP † by ≥50% and elimination of fever for >48 hours • Change from baseline DAS ‡ score, modified Pouchet score, and DAS - CRP • Change in CRP, ferritin, and ESR § • Change in IL - 18 levels • Safety and tolerability • Active AOSD as measured by high fever, elevated CRP and ferritin • Failed on NSAIDS and corticosteroids AVTX - 007 14 mg/kg (max 500 mg) q4wks (n=6) 12 weeks AOSD initial data anticipated 4Q 2021 AVTX - 007 Treatment of Adult - Onset Still’s Disease Potential Best - in - Class and First - in - Class Anti - IL - 18* mAb Multicenter, Phase 1b Study of AVTX - 007 in patients with active adult - onset Still’s disease Proof - of - Concept Trial Design Inclusion Criteria

42 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. 42 Phase 2 - ready, dual mTORC 1/2 small - molecule inhibitor for Complex Lymphatic Malformations AVTX - 006
43 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. High - Potency, Second - Generation, Dual Inhibitor of mTORC1/2 • In - licensed from Astellas • Phase 2 – ready asset – 4 - week nonclinical tox studies completed – Previously studied in Phase 1 MAD* (n=128) – Development discontinued upon determination that target efficacious doses were above MTD † (30mg QD) 1 – Significantly lower doses than MTD likely required to treat complex lymphatic malformations • Dual mTOR ‡ inhibitor maximizes impact of mTOR blockade, as mTORC2 is insensitive to rapalogs – Orally available, ATP - competitive kinase inhibitor Α – IC 50 ¶ = 22 nM and 65 nM for mTORC1 and mTORC2, respectively 2 *MAD, multiple ascending dose; † MTD, maximum tolerated dose; ‡ mTOR, mammalian target of rapamycin; Α ATP, adenosine triphosphate; ¶ IC, half maximal inhibitory concentration. 1. Mateo J et al. Br J Cancer. 2016;114(8):889 - 896. 2. Bhagwat SV et al. Mol Cancer Ther . 2011; 10(8):1394 - 1406. Potential for Improved Efficacy and Tolerability
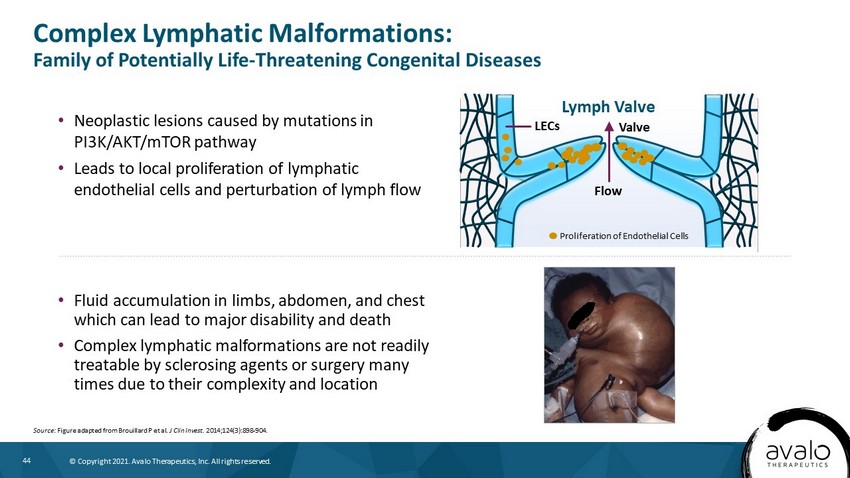
44 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • Neoplastic lesions caused by mutations in PI3K/AKT/mTOR pathway • Leads to local proliferation of lymphatic endothelial cells and perturbation of lymph flow Complex Lymphatic Malformations: Family of Potentially Life - Threatening Congenital Diseases Source : Figure adapted from Brouillard P et al. J Clin Invest. 2014;124(3):898 - 904. • Fluid accumulation in limbs, abdomen, and chest which can lead to major disability and death • Complex lymphatic malformations are not readily treatable by sclerosing agents or surgery many times due to their complexity and location Flow Valve LECs Lymph Valve Proliferation of Endothelial Cells
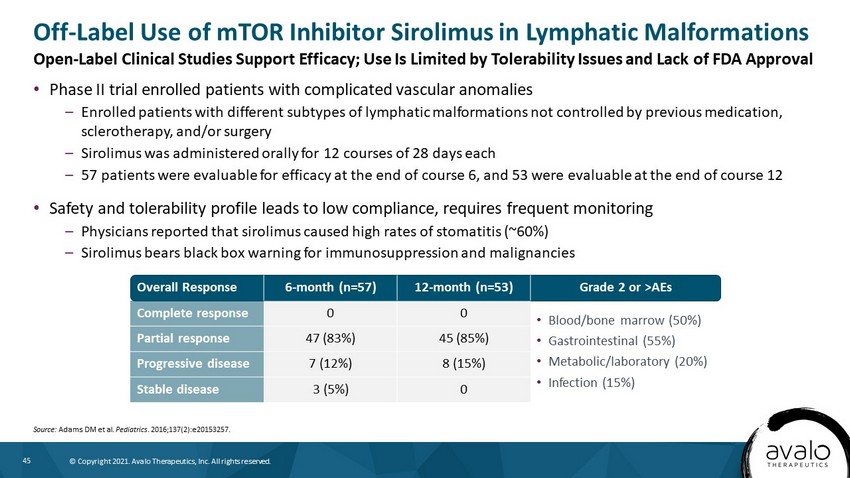
45 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • Phase II trial enrolled patients with complicated vascular anomalies – Enrolled patients with different subtypes of lymphatic malformations not controlled by previous medication, sclerotherapy, and/or surgery – Sirolimus was administered orally for 12 courses of 28 days each – 57 patients were evaluable for efficacy at the end of course 6, and 53 were evaluable at the end of course 12 • Safety and tolerability profile leads to low compliance, requires frequent monitoring – Physicians reported that sirolimus caused high rates of stomatitis (~60%) – Sirolimus bears black box warning for immunosuppression and malignancies Off - Label Use of mTOR Inhibitor Sirolimus in Lymphatic Malformations Source: Adams DM et al. Pediatrics . 2016;137(2):e20153257. Overall Response 6 - month (n=57) 12 - month (n=53) Grade 2 or >AEs Complete response 0 0 • Blood/bone marrow (50%) • Gastrointestinal (55%) • Metabolic/laboratory (20%) • Infection (15%) Partial response 47 (83%) 45 (85%) Progressive disease 7 (12%) 8 (15%) Stable disease 3 (5%) 0 Open - Label Clinical Studies Support Efficacy; Use Is Limited by Tolerability Issues and Lack of FDA Approval
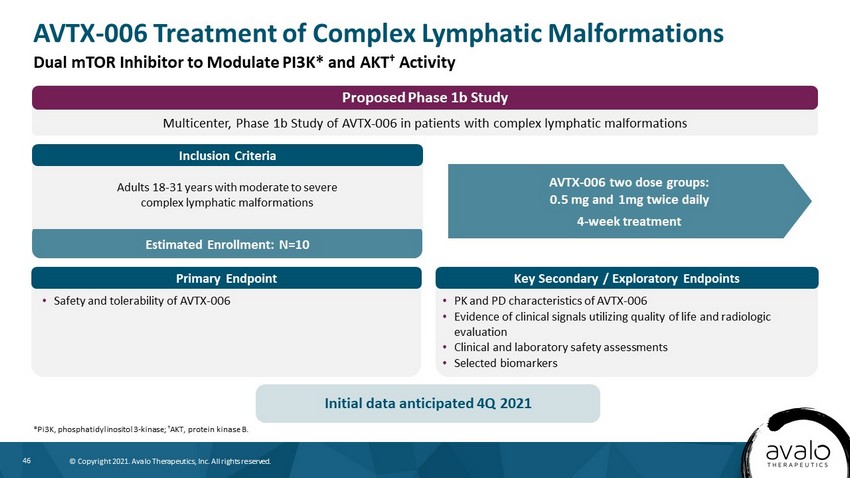
46 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Estimated Enrollment: N=10 AVTX - 006 Treatment of Complex Lymphatic Malformations *Pi3K, phosphatidylinositol 3 - kinase; † AKT, protein kinase B. Dual mTOR Inhibitor to Modulate PI3K* and AKT † Activity AVTX - 006 two dose groups: 0.5 mg and 1mg twice daily 4 - week treatment • Safety and tolerability of AVTX - 006 • PK and PD characteristics of AVTX - 006 • Evidence of clinical signals utilizing quality of life and radiologic evaluation • Clinical and laboratory safety assessments • Selected biomarkers Adults 18 - 31 years with moderate to severe complex lymphatic malformations Initial data anticipated 4Q 2021 Multicenter, Phase 1b Study of AVTX - 006 in patients with complex lymphatic malformations Proposed Phase 1b Study Inclusion Criteria Key Secondary / Exploratory Endpoints Primary Endpoint

47 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. 47 Monosaccharide therapy for Congenital Disorders of Glycosylation (CDGs) AVTX - 800s
48 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. • Glycosylation is essential for protein structure & function, particularly for circulating proteins and enzymes such as hormones and coagulation factors • Currently approximately 150 CDGs identified • Due to a genetic mutation, CDG patients lack the ability to synthesize functioning glycoproteins • Life - threatening multi - system diseases: failure to thrive, developmental delay, hypotonia, neurologic abnormalities, hepatic disease, and coagulopathy • Administration of therapeutic doses of specific monosaccharides targeted to specific CDGs can partially restore impaired glycoprotein production resulting in a meaningful clinical benefit – PGM1 - CDG: D - galactose supplementation 1 – MPI - CDG: D - mannose supplementation 2 – LAD - II (SLC35C1 - CDG): L - fucose supplementation 3 Congenital Disorders of Glycosylation (CDGs): Life - Threatening, Ultra - Rare Disease 1. Wong et al. Genet Med. 2017;19(11):1226 - 1235. 2. Harms et al. Acta Paediatr. 2002;91(10):1065 - 1072. 3. Marquardt et al. Blood . 1991;94(12):3976 - 3985. Impaired Glycoprotein Production and Function Restored With Therapeutic Dose of Monosaccharide Therapies
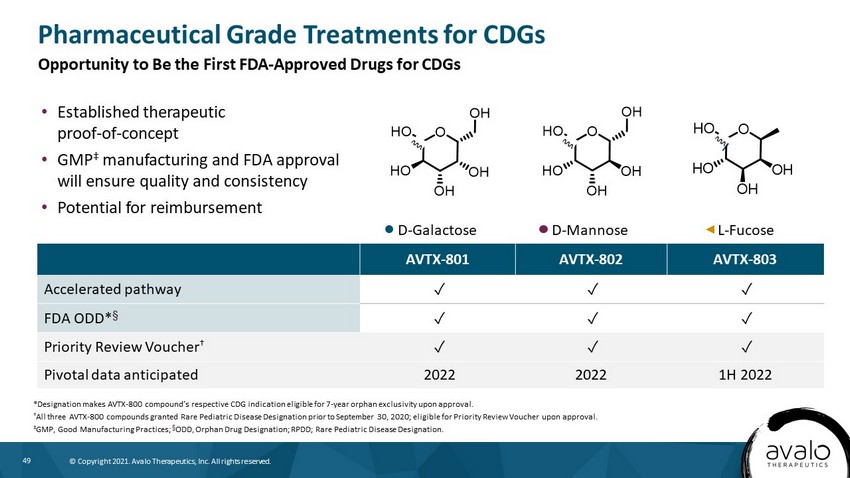
49 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. D - Mannose D - Galactose L - Fucose OHO OH OH HO OH O HO OH OH HO OH AVTX - 801 AVTX - 802 AVTX - 803 Accelerated pathway ض ض ض FDA ODD* † ض ض ض Priority Review Voucher † ض ض ض Pivotal data anticipated 2022 2022 1H 2022 Pharmaceutical Grade Treatments for CDGs *Designation makes AVTX - 800 compound’s respective CDG indication eligible for 7 - year orphan exclusivity upon approval. † All three AVTX - 800 compounds granted Rare Pediatric Disease Designation prior to September 30, 2020; eligible for Priority Revie w Voucher upon approval. ‡ GMP, Good Manufacturing Practices; † ODD, Orphan Drug Designation; RPDD; Rare Pediatric Disease Designation. • Established therapeutic proof - of - concept • GMP ‡ manufacturing and FDA approval will ensure quality and consistency • Potential for reimbursement Opportunity to Be the First FDA - Approved Drugs for CDGs

50 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. 50 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Key Financial Information
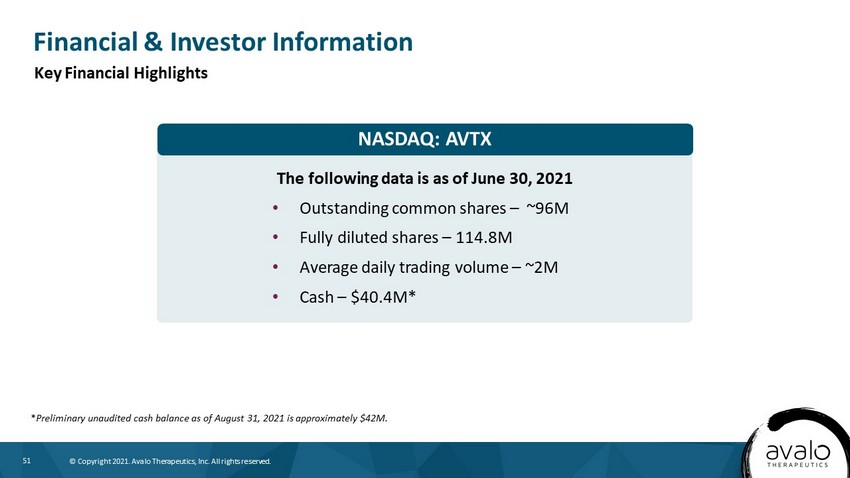
51 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. NASDAQ: AVTX Financial & Investor Information The following data is as of June 30, 2021 • Outstanding common shares – ~96M • Fully diluted shares – 114.8M • Average daily trading volume – ~2M • Cash – $40.4M* Key Financial Highlights * Preliminary unaudited cash balance as of August 31, 2021 is approximately $42M.

52 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. Select Management Team Members Michael Cola | Chief Executive Officer, Chairman of the Board • Former President of Specialty Pharmaceuticals, Shire plc • Senior Management, AstraMerck and AstraZeneca plc Garry Neil, MD | Chief Scientific Officer • Former Corporate VP of Science & Technology, Johnson & Johnson • Former Group President, Johnson & Johnson Pharmaceutical Research and Development • Former VP of Clinical Research, AstraZeneca plc and Merck KGaA H. Jeffrey Wilkins, MD | Chief Medical Officer • Former VP, Worldwide Clinical Research, Inflammation/Oncology at Cephalon and SVP of Clinical Development with Ception Therapeutics • Former VP of Discovery Medicine for GSK’s Center of Excellence in External Drug Disc overy Proven Track Record in Drug Development and Commercialization

53 © Copyright 2021. Avalo Therapeutics, Inc. All rights reserved. NASDAQ:AVTX www.avalotherapeutics.com